
文章信息
- 宋琦, 李晓明, 胡雪怡, 杨遂群, 王斌贵. 2019.
- SONG Qi, LI Xiao-ming, HU Xue-yi, YANG Sui-qun, WANG Bin-gui. 2019.
- 海鞘内生真菌棒曲霉Aspergillus clavatus AS-107的化学成分研究
- Chemical constituents of an endophytic fungus derived from ascidian Aspergillus clavatus AS-107
- 海洋科学, 43(2): 12-17
- Marine Sciences, 43(2): 12-17.
- http://dx.doi.org/10.11759/hykx20190321005
-
文章历史
- 收稿日期:2018-08-05
- 修回日期:2018-12-11
2. 中国科学院大学, 北京 100049;
3. 中国科学院海洋大科学研究中心, 山东 青岛 266071
2. University of the Chinese Academy of Sciences, Beijing 100049, China;
3. Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
近年来, 海洋微生物来源的天然产物因结构新颖多样、生物活性显著而受到化学家、生物学家和药物学家的关注[1]。海鞘、海绵等海洋无脊椎动物分布广泛, 属于发现天然产物最多的海洋动物, 然而越来越多的证据表明, 许多从海洋无脊椎动物中分离得到具有生物活性的天然产物其真正生产者并不是动物本身, 而是它体内的微生物[2]。海鞘为滤食性底栖海洋生物, 因而体内聚集了较多的微生物, 通过分离海鞘体内的微生物并获取天然产物是丰富天然产物研究内容的重要途径[3-4]。
本文报道分离自海鞘新鲜组织中的棒曲霉A. clavatus AS-107的代谢产物。该菌株在PDA培养基上呈青绿色菌丝体和孢子。对其进行大米固体培养基静置培养发酵的方式并提取得到其次级代谢产物粗提物, 后借助多种色谱分离方法获得7个单体化合物, 运用核磁共振技术鉴定结构(图 1), 分别是norquinadoline A (1)[5]、quinadoline A (2)[5]、epi- fiscalin D (3)[6]、fiscalin B (4)[7-8]、quinadoline B (5)[9]、prelapatin B (6)[5, 10-11]和tryptoquivalines L (7)[12]。对所有化合物进行了抗菌活性测试。
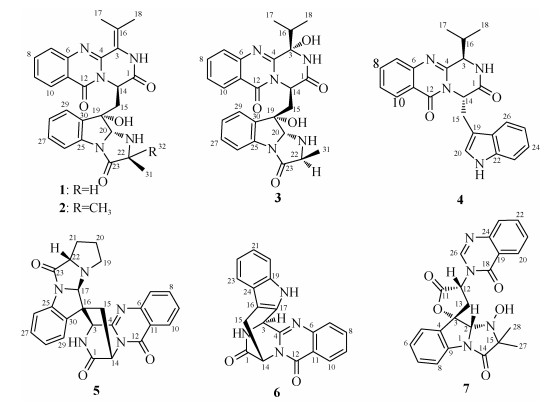 |
| 图 1 分离获得的化合物1-7的化学结构 Fig. 1 Chemical structures of the isolated compounds 1-7 |
本实验所用仪器、耗材和试剂如表 1所示。
| 仪器/试剂名称 | 型号/规格 | 生产厂家 |
| 自动旋光仪 | Optical Activity Limited AA-55 | 英国Optical Activity公司 |
| 核磁共振仪 | Bruker-Avance 500 | 瑞士Bruker公司 |
| 紫外-可见光谱仪 | Gold Spectrumlab 54 | 上海棱光技术有限公司 |
| 薄层色谱硅胶 | GF254 | 青岛海洋化工厂分厂 |
| 正相硅胶 | 100~200目、200~300目 | 青岛海洋化工厂分厂 |
| 葡聚糖凝胶 | Sephadex LH-20(18~110 mm) | 美国Pharmacia公司 |
| 96孔板 | REF3599 | 康宁生命科学有限公司 |
| 有机溶剂 | 工业级, 使用前经重新蒸馏 | 青岛新宇田化工有限公司 |
(1) 菌株:菌株AS-107分离自2016年10月采集于印度尼西亚海域的海鞘。通过形态学观察法和ITS序列系统发育分析法[13], 将该菌鉴定为棒曲霉Aspergillus clavatus, 现存放于中国科学院海洋研究所实验海洋生物学重点实验室。
(2) 菌株培养:菌种保存在含有20%甘油-水的保菌管中, 并于–80℃下冷藏。按照我们之前报道的发酵方法[14], 选用大米固体培养基作为发酵培养基, 在1 L三角瓶中加入大米70 g、蛋白胨0.3 g、玉米粉0.1 g、天然过滤海水100 mL。培养基经高温高压灭菌后接入菌种, 于28℃恒温、自然光条件下静置培养30d。
1.3 菌株次级代谢产物的提取与分离规模发酵100瓶, 用乙酸乙酯对发酵产物进行萃取, 静置沉降后, 取上层有机相经减压浓缩得到粗提物115.7 g。对其进行硅胶真空柱层析, 用有机溶剂(PE-EtOAc、CH2Cl2-MeOH)作为洗脱剂, 极性由小到大进行梯度洗脱。对粗分离后的样品进行TLC和HPLC检测分析, 合并得到9个组分(Fr.1~9)。
Fr. 5 (PE-EtOAc 1︰1, 12.3 g)经反相硅胶柱层析、正相硅胶柱层析(CH2Cl2︰EtOAc=100︰1~10︰1)、葡聚糖凝胶SephadexLH-20(MeOH)柱层析分离得到化合物4 (8.8 mg)、5(35.2 mg); Fr. 6 (CH2Cl2-MeOH 40: 1, 14.5 g)经反相硅胶柱层析、pTLC、葡聚糖凝胶SephadexLH-20(MeOH)分离得到化合物2 (18.9 mg)、7 (8.8 mg); Fr. 7 (CH2Cl2-MeOH 20︰1, 30.4 g)经硅胶真空柱层析(CH2Cl2︰CH3COCH3= 20︰1~1︰1)、反相硅胶柱层析、正相硅胶柱层析(CH2Cl2︰MeOH= 200︰1~10︰1)、葡聚糖凝胶SephadexLH-20(MeOH)柱层析和pTLC分离得到化合物6 (5.6 mg)、1 (18.4 mg)、3 (10.8 mg)。
1.4 抑菌活性MIC测试(1) 病原指示菌:选用了5株水产致病细菌, 分别为嗜水气单胞菌(Aeromonas hydrophilia)、副溶血性弧菌(Vibrio parahaemolyticus)、鳗弧菌(V. anguillarum)、哈氏弧菌(V. harveyi)和铜绿假单胞菌(Pseudomonas aeruginosa)。以上菌株均来自于中国科学院海洋研究所实验海洋生物学重点实验室。
(2) 指示菌菌悬液制备:将指示菌接种在LB培养基表面, 在37℃环境中静置培养24 h, 后取适量的无菌0.85%氯化钠溶液洗涤培养基表面的菌落, 用无菌刮刀将其轻轻刮下。吸取适量指示菌悬液至无菌玻璃试管中, 并用0.85%氯化钠溶液将菌悬液稀释至0.5麦氏浊度(相当于1.5×108 CFU/mL), 密封好备用。
(3) 样品的配制:称取适量样品, 配置得到6组浓度依次减半的样品溶液(1280, 640, 320, 160, 80, 40 μg/mL)。阳性对照为氯霉素, 配制浓度梯度为80, 40, 20, 10, 5, 2.5 μg/mL。
(4) 抑菌活性MIC测试:活性实验参照我们实验室之前的研究方法[15, 16]。进行无菌操作, 使用移液枪将提前配制好的麦氏浊度为0.5的指示菌悬液依次加入到无菌的96孔聚苯乙烯板中, 每孔95 μL。再以相同方式将梯度浓度的样品溶液和阳性对照分别加入到96孔板中, 每孔5 μL。将96孔板密封好, 并轻轻震荡混匀, 置于37℃恒温培养箱中, 24 h后检测实验结果。利用酶标仪(波长为600 nm)测定每孔的吸光值, 有效MIC值为该样品完全抑制指示菌生长时的最低浓度。
2 单体化合物结构鉴定和抗细菌活性结果 2.1 单体化合物结构鉴定化合物1:白色固体, 13C NMR (125 MHz, DMSO- d6) δC: 166.8 (C, C-1), 121.4 (C, C-3), 146.8 (C, C-4), 146.8 (C, C-6), 127.2 (CH, C-7), 134.6 (CH, C-8), 126.9 (CH, C-9), 126.3 (CH, C-10), 119.6 (C, C-11), 159.7 (C, C-12), 52.0 (CH, C-14), 37.9 (CH2, C-15), 131.2 (C, C-16), 21.1 (CH3, C-17), 21.4 (CH3, C-18), 75.1(C, C-19), 81.3 (CH, C-20), 60.0 (CH, C-22), 174.6 (C, C-23), 137.8 (C, C-25), 114.9 (CH, C-26), 129.4 (CH, C-27), 124.5 (CH, C-28), 124.5 (CH, C-29), 137.9 (C, C-30), 18.1 (CH3, C-31); 1H NMR (500 MHz, DMSO-d6) δH: 10.13 (1H, s, NH-2), 7.67 (1H, d, J = 8.1 Hz, H-7), 7.85 (1H, t, J = 7.7 Hz, H-8), 7.55 (1H, t, J = 7.5 Hz, H-9), 8.17 (1H, d, J = 7.6 Hz, H-10), 5.50 (1H, dd, J = 7.9, 5.4 Hz, H-14), 3.80 (1H, d, J = 7.1 Hz, H-22), 2.53 (1H, m, H-15), 2.36 (1H, m, H-15), 1.97 (3H, s, H-17), 2.33 (3H, s, H-18), 5.30 (1H, s, H-20), 7.34 (1H, m, H-26), 7.34 (1H, m, H-27), 7.11 (1H, dd, J = 7.1, 1.9 Hz, H-28), 7.34 (1H, m, H-29), 1.29 (3H, d, J = 7.1 Hz, H-31); UV (MeOH) λmax (log ε) 209 (4.55), 222 (4.39), 348 (3.99) nm。其波谱数据和紫外吸收与norquinadoline A[5]的文献报道一致。且化合物1的比旋光度为[α]D25–4.1 (c 0.10, MeOH)与文献报道的[α]D25–2.7 (c 0.10, MeOH)接近, 说明两者的绝对构型也相同, 由此将化合物1的结构鉴定为norquinadoline A。
化合物2:白色固体, 13C NMR (125 MHz, DMSO- d6) δC: 166.9 (C, C-1), 121.5 (C, C-3), 146.8 (C, C-4), 146.9 (C, C-6), 127.1 (CH, C-7), 134.5 (CH, C-8), 126.9 (CH, C-9), 126.2 (CH, C-10), 119.6 (C, C-11), 159.8 (C, C-12), 52.3 (CH, C-14), 38.0 (CH2, C-15), 130.7 (C, C-16), 21.1 (CH3, C-17), 21.5 (CH3, C-18), 74.4 (C, C-19), 78.6 (CH, C-20), 64.5(C, C-22), 175.2 (C, C-23), 137.8 (C, C-25), 114.4 (CH, C-26), 129.6 (CH, C-27), 124.7 (CH, C-28), 124.4 (CH, C-29), 137.5 (C, C-30), 24.6 (CH3, C-31), 24.1 (CH3, C-32); 1H NMR (500 MHz, DMSO-d6)δH: 10.12 (1H, s, NH-2), 7.66 (1H, d, J = 8.1 Hz, H-7), 7.84 (1H, m, H-8), 7.54 (1H, t, J = 7.5 Hz, H-9), 8.16 (1H, dd, J= 7.9, 0.9 Hz, H-10), 5.47 (1H, dd, J = 8.3, 4.5 Hz, H-14), 2.60 (1H, dd, J= 14.5, 4.5 Hz, H-15), 2.44 (1H, dd, J = 14.5, 8.4 Hz, H-15), 1.96 (3H, s, H-17), 2.31 (3H, s, H-18), 5.59 (1H, s, 19-OH), 5.09 (1H, d, J = 8.8 Hz, H-20), 7.34 (1H, m, H-26), 7.35 (1H, m, H-27), 7.11 (1H, m, H-28), 7.39 (1H, d, J = 7.6 Hz, H-29), 1.21 (3H, s, H-31), 1.15 (3H, s, H-32); UV (MeOH) λmax (log ε) 209 (4.62), 227 (4.43), 350 (4.00) nm。其波谱数据和紫外吸收与已知化合物quinadoline A[5]的相关数据一致。同时化合物2的比旋光度为[α]D25–41.4 (c 0.10, MeOH)与文献报道的[α]D25–32.0 (c 0.10, MeOH)接近, 故将该化合物鉴定为quinadoline A。
化合物3:白色固体, 13C NMR (125 MHz, DMSO- d6) δC: 170.3 (C, C-1), 84.2 (C, C-3), 151.2 (C, C-4), 146.3 (C, C-6), 127.3 (CH, C-7), 134.8 (CH, C-8), 127.3 (CH, C-9), 126.3 (CH, C-10), 120.1 (C, C-11), 160.2 (C, C-12), 52.0 (CH, C-14), 41.1 (CH2, C-15), 33.6 (CH, C-16), 14.4 (CH3, C-17), 17.5 (CH3, C-18), 75.3(C, C-19), 81.5 (CH, C-20), 59.8 (CH, C-22), 174.8 (C, C-23), 138.4 (C, C-25), 115.0 (CH, C-26), 124.6 (CH, C-27), 124.5 (CH, C-28), 129.2 (CH, C-29), 137.9 (C, C-30), 18.0 (CH3, C-31); 1H NMR (500 MHz, DMSO-d6) δH: 7.68 (1H, d, J = 8.1 Hz, H-7), 7.84 (1H, dd, J = 11.9, 4.9 Hz, H-8), 7.56 (1H, t, J = 7.5 Hz, H-9), 8.16 (1H, dd, J = 7.9, 1.1 Hz, H-10), 5.46 (1H, dd, J= 8.2, 3.9 Hz, H-14), 2.77 (1H, d, J = 8.3 Hz, H-15), 2.69 (1H, m, H-15), 3.00 (1H, d, J = 7.0 Hz, H-16), 1.11 (3H, d, J = 7.1 Hz, H-17), 0.9 (3H, d, J = 6.7 Hz, H-18); 5.36 (1H, s, H-20), 3.77 (1H, d, J = 7.0 Hz, H-22), 7.30 (1H, m, H-26), 7.08 (1H, td, J = 7.4, 1.3 Hz, H-27), 7.38 (1H, d, J = 7.6 Hz, H-28), 7.30 (1H, m, H-29), 1.27 (3H, d, J= 7.1 Hz, H-31); UV (MeOH) λmax (log ε) 203 (4.69), 226 (4.54), 304 (3.61), 316 (3.53) nm。其波谱数据和紫外吸收与epi-fiscalin D[6]的文献报道一致。且化合物3的比旋光度为[α]D25–162.5 (c 0.10, MeOH)与已知化合物的[α]22D–175.9 (c 0.06, MeOH)接近, 故将该化合物鉴定为epi-fiscalin D。
化合物4:黄色油状液体, 13C NMR (125 MHz, CDCl3) δC: 169.5 (C, C-1), 58.2 (CH, C-3), 150.4 (C, C-4), 147.2 (C, C-6), 127.1 (CH, C-7), 134.8 (CH, C-8), 127.3 (CH, C-9), 127.0 (CH, C-10), 120.4 (C, C-11), 161.0 (C, C-12), 57.0 (CH, C-14), 27.5 (CH2, C-15), 29.6 (CH, C-16), 14.9 (CH3, C-17), 19.0 (CH3, C-18), 109.6 (CH, C-19), 123.7 (CH, C-20), 136.2 (C, C-22), 111.2 (CH, C-23), 122.7 (CH, C-24), 120.2 (CH, C-25), 118.9 (CH, C-26), 127.4 (C, C-27); 1H NMR (500 MHz, CDCl3) δH: 5.66 (1H, m, NH-2), 2.63 (1H, m, H-3), 7.54 (1H, dd, J = 15.6, 7.6 Hz, H-7), 7.77 (1H, t, J = 6.9 Hz, H-8), 7.54 (1H, dd, J = 15.6, 7.6 Hz, H-9), 8.37 (1H, d, J = 7.9 Hz, H-10), 5.66 (1H, m, H-14), 3.74 (1H, dd, J = 15.0, 2.3 Hz, H-15), 3.64 (1H, dd, J = 15.0, 5.3 Hz, H-15), 2.69 (1H, d, J = 1.9 Hz, H-16), 0.64 (3H, t, J = 6.7 Hz, H-17), 0.64 (3H, t, J = 6.7 Hz, H-18), 6.60 (1H, d, J = 1.9 Hz, H-20), 8.11 (1H, s, NH-21), 7.27 (1H, m, H-23), 7.12 (1H, t, J = 7.2 Hz, H-24), 6.93 (1H, t, J = 7.5 Hz, H-25), 7.43 (1H, d, J = 8.1 Hz, H-26); UV (MeOH) λmax (log ε) 219 (4.67), 274 (4.06), 290 (3.91), 305 (3.58), 319 (3.47) nm。其波谱数据和紫外吸收与fiscalin B[7-8]文献报道一致。且化合物4的比旋光度为[α]D25–221.5 (c 0.10, MeOH)与已知化合物的[α]D25–248.1 (c 0.06, CHCl3)接近, 故将化合物4鉴定为fiscalin B。
化合物5:白色固体, 13C NMR (125 MHz, CDCl3) δC: 170.8 (C, C-1), 60.0 (CH, C-3), 151.3 (C, C-4), 146.8 (C, C-6), 126.9 (CH, C-7), 135.0 (CH, C-8), 127.7 (CH, C-9), 126.9 (CH, C-10), 120.6 (C, C-11), 159.0 (C, C-12), 53.0 (CH, C-14), 33.6 (CH2, C-15), 53.2 (C, C-16), 91.9 (CH, C-17), 56.4 (CH2, C-19), 24.7 (CH2, C-20), 29.4 (CH2, C-21), 69.5 (CH, C-22), 174.2 (C, C-23), 137.1 (C, C-25), 116.5 (CH, C-26), 129.2 (CH, C-27), 126.7 (CH, C-28), 123.9 (CH, C-29), 137.1 (C, C-30); 1H NMR (500 MHz, CDCl3) δH: 4.66 (1H, s, H-3), 7.73 (1H, d, J = 8.0 Hz, H-7), 7.86 (1H, m, H-8), 7.60 (1H, m, H-9), 8.33 (1H, dd, J= 8.0, 1.1 Hz, H-10), 5.71 (1H, dd, J = 3.7, 1.6 Hz, H-14), 3.01 (1H, dd, J = 13.9, 3.9 Hz, H-15), 1.86 (1H, m, H-15), 5.02 (1H, d, J = 1.0 Hz, H-17), 2.45 (1H, m, H-19), 1.82 (1H, m, H-19), 1.50 (3H, m, H-20), 1.50 (3H, m, H-20), 1.97 (1H, m, H-21), 1.82 (1H, m, H-21), 3.85 (1H, m, H-22), 7.47 (1H, t, J = 7.6 Hz, H-26), 7.37 (1H, td, J = 7.8, 1.0 Hz, H-27), 7.24 (1H, td, J = 7.7, 1.0 Hz, H-28), 7.60 (1H, m, H-29); UV (MeOH) λmax (log ε) 225 (4.56), 266 (4.10), 276 (2.08), 302 (3.64), 314 (3.54)nm。其波谱数据和紫外吸收与已知化合物quinadoline B[9]的相关数据一致。且化合物5的比旋光度为[α]D25–58.8 (c 0.17, MeOH)与文献报道的[α]22D–44.7 (c 0.01, MeOH)接近, 故将该化合物鉴定为quinadoline B。
化合物6:白色固体, 13C NMR (125 MHz, DMSO- d6) δC: 169.3 (C, C-1), 51.1 (CH, C-3), 152.2 (C, C-4), 147.0 (C, C-6), 127.0 (CH, C-7), 134.8 (CH, C-8), 127.2 (CH, C-9), 126.4 (CH, C-10), 119.4 (C, C-11), 159.2 (C, C-12), 54.4 (CH, C-14), 25.5 (CH2, C-15), 105.0 (C, C-16), 131.5 (C, C-17), 134.8 (C, C-19), 111.7 (CH, C-20), 122.2 (CH, C-21), 120.3 (CH, C-22), 118.2 (CH, C-23), 127.2 (C, C-24); 1H NMR (500 MHz, DMSO-d6) δH: 9.71 (1H, d, J = 5.0 Hz, NH-2), 5.32 (1H, d, J = 5.4 Hz, H-3), 7.63 (1H, d, J = 7.9 Hz, H-7), 7.81 (1H, m, H-8), 7.53 (1H, m, H-9), 8.15 (1H, dd, J = 8.0, 1.2 Hz, H-10), 5.66 (1H, s, H-14), 3.43 (1H, dd, J = 17.3, 2.8 Hz, H-15), 3.24 (1H, dd, J = 17.3, 4.4 Hz, H-15), 11.53 (1H, s, NH-18), 7.38 (1H, d, J = 8.1 Hz, H-20), 7.11 (1H, ddd, J = 8.1, 7.1, 1.0 Hz, H-21), 6.99 (1H, m, H-22), 7.42 (1H, d, J = 7.9 Hz, H-23); UV (MeOH) λmax (log ε) 220 (4.69), 269 (4.10), 292 (4.14) nm。其波谱数据和紫外吸收与prelapatin B[5, 10-11]的文献报道一致。且化合物6的比旋光度为[α]D25 182.9 (c 0.10, MeOH)与已知化合物的[α]D25 173.5 (c 0.06, EtOAc)接近, 故将该化合物鉴定为prelapatin B。
化合物7:白色固体, 13C NMR (125 MHz, DMSO- d6) δC: 186.4 (CH, C-2), 83.5 (C, C-3), 132.5 (C, C-4), 126.0 (CH, C-5), 125.3 (CH, C-6), 131.7 (CH, C-7), 114.7 (CH, C-8), 138.2 (C, C-9), 171.2 (C, C-11), 56.8 (CH, C-12), 34.1 (CH2, C-13), 71.3 (C, C-14), 70.5 (C, C-15), 159.7 (C, C-18), 121.4 (C, C-19), 126.1 (CH, C-20), 127.6 (CH, C-21), 135.0 (CH, C-22), 127.3 (CH, C-23), 147.6 (C, C-24), 147.6 (C, C-26), 16.5 (CH3, C-27), 22.8(CH3, C-28); 1H NMR (500 MHz, DMSO-d6)δH: 5.24 (1H, s, H-2), 7.91 (1H, m, H-5), 7.37 (1H, d, J= 7.6 Hz, H-6), 7.55 (1H, d, J = 7.3 Hz, H-7), 7.50 (1H, d, J = 7.7 Hz, H-8), 5.58 (1H, t, J = 10.1 Hz, H-12), 3.44 (1H, m, H-13), 3.05 (1H, m, H-13), 9.16 (1H, s, 16-OH), 8.25 (1H, m, H-20), 7.64 (1H, t, J = 7.5 Hz, H-21), 7.81 (1H, d, J = 7.6 Hz, H-22), 7.77 (1H, d, J = 8.0 Hz, H-23), 8.60 (1H, s, H-26), 1.35 (3H, s, H-27), 1.25 (3H, s, H-28); UV (MeOH) λmax (log ε) 225 (4.49), 254 (4.25), 276 (3.70), 303 (3.26), 313 (3.15) nm。其波谱数据和紫外吸收与tryptoquivalines L[12]的文献报道一致。且化合物7的比旋光度为[α]D25–142.4 (c 0.10, MeOH)与文献报道的[α]20D –154.0 (c 0.12, MeOH)接近, 说明两者绝对构型也相同, 故将化合物7鉴定为tryptoquivalines L。
2.2 抗细菌活性结果实验结果表明化合物2对嗜水气单胞菌(Aeromonas hydrophilia)具有一定的抑制作用, 其MIC值为16 μg/mL; 化合物6对副溶血性弧菌(Vibrio parahaemolyticus)、嗜水气单胞菌(Aeromonas hydrophilia)和哈氏弧菌(V. harveyi)有一定的抑制活性, 其MIC值分别为16、32和32 μg/mL; 此外化合物3对鳗弧菌(V. anguillarum)具有微弱抑制作用, 其MIC值为32 μg/mL。根据实验结果可知, 对于同类化合物1, 2和3, C-3的羟基和C-22的甲基取代均可以提高化合物的抗菌活性。
| [1] |
Chen G, Wang H F, Pei Y H. Secondary metabolites from marine-derived microorganisms[J]. Journal of Asian Natural Products Research, 2014, 16(1): 105-122. DOI:10.1080/10286020.2013.855202 |
| [2] |
Unson M D, Holland N D, Faulkner D J. A brominated secondary metabolite synthesized by the cyanobacterial symbiont of a marine sponge and accumulation of the crystalline metabolite in the sponge tissue[J]. Marine Biology, 1994, 119(1): 1-11. DOI:10.1007/BF00350100 |
| [3] |
Garo E, Starks C M, Jensen P R, et al. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens[J]. Journal of Natural Products, 2003, 66(3): 423-426. |
| [4] |
Zubía E, Ortega M J, Salvá J. Natural products chemistry in marine ascidians of the genus Aplidium[J]. Mini-Reviews in Organic Chemistry, 2005, 2(4): 389-399. |
| [5] |
Peng J X, Lin T, Wang W, et al. Antiviral alkaloids produced by the mangrove-derived fungus Cladosporium sp. PJX-41[J]. Journal of Natural Products, 2013, 76(6): 1133-1140. DOI:10.1021/np400200k |
| [6] |
Qian S Y, Yang C L, Khan A, et al. New pyrazinoquinazoline alkaloids Isolated from a culture of Stenotrophomonas maltophilia QB-77[J]. Natural Product Research, 2019, 33(9): 1387-1391. DOI:10.1080/14786419.2018.1475381 |
| [7] |
Solida L, Diana I S P R, Anake K, et al. Antitumor activity of quinazolinone alkaloids inspired by marine natural products[J]. Marine Drugs, 2018, 16(8): 261. DOI:10.3390/md16080261 |
| [8] |
Fujimoto H, Negishi E, Yamaguchi K, et al. Isolation of new tremorgenic metabolites from an ascomycete, Corynascus setosus[J]. Chemical & Pharmaceutical Bulletin, 1996, 44(10): 1843-1848. |
| [9] |
Koyama N, Inoue Y, Sekine M, et al. Relative and absolute stereochemistry of quinadoline B, an inhibitor of lipid droplet synthesis in macrophages[J]. Organic Letters, 2008, 10(22): 5273-5276. DOI:10.1021/ol802089p |
| [10] |
Lin T, Tan T, Liu T X, et al. Study on the secondary metabolites and their anti-tumor activity from mangroves fungi PJX-41[J]. Journal of Nanjing Agricultural University, 2013, 36(3): 117-123. |
| [11] |
Walker S J, Hart D J. Synthesis of (-)-lapatin B[J]. Tetrahedron Letters, 2007, 48(35): 6214-6216. DOI:10.1016/j.tetlet.2007.06.089 |
| [12] |
Suradet B, Angsumarn C, Leka M, et al. Sartorymensin, a new indole alkaloid, and new analogues of tryptoquivaline and fiscalins produced by Neosartorya siamensis (KUFC 6349)[J]. Tetrahedron, 2012, 68(15): 3253-3262. DOI:10.1016/j.tet.2012.02.024 |
| [13] |
Wang S, Li X M, Teuscher F, et al. Chaetopyranin, a benzaldehyde derivative and other related metabolites from Chaetomium globosum, an endophytic fungus derived from marine red alga Polysiphonia urceolata[J]. Journal of Natural Products, 2006, 69(11): 1622-1625. DOI:10.1021/np060248n |
| [14] |
李莉, 李晓明, 李洪雷, 等. 海鞘内生真菌焦曲霉Aspergillus ustus TK-5的化学成分研究[J]. 海洋科学, 2018, 42(5): 130-137. Li Li, Li Xiaoming, Li Honglei, et al. Chemical constituents of Aspergillus ustus TK-5, an endophytic fungus derived from the ascidian Herdmania momus[J]. Marine Sciences, 2018, 42(5): 130-137. |
| [15] |
孙好芬.两株热带马尾藻内生真菌次生代谢产物研究[D].青岛: 中国科学院海洋研究所, 2010. Sun Haofen. Study on secondary metabolites of two endophytic fungal strains from tropical Sargassum species[D]. Qingdao: Institute of Oceanology, Chinese Academy of Sciences, 2010. |
| [16] |
王佳宁, 李晓明, 张鹏, 等. 日本仙菜来源内生真菌Aspergillus versicolor EN-298化学成分研究[J]. 海洋科学, 2015, 39(6): 94-98. Wang Jianing, Li Xiaoming, Zhang Peng, et al. Chemical constituents of Aspergillus versicolor EN-298, an endophytic fungus derived from the marine alga Ceramium japonicum.[J]. Marine Sciences, 2015, 39(6): 94-98. |
 2019, Vol. 43
2019, Vol. 43


