文章信息
- 彭壮壮, 邹玉霞, 刘琰, 梁少帅, 王雯祥, 王丽娟, 邹聪聪, 周顺, 尤锋. 2021.
- PENG Zhuang-zhuang, ZOU Yu-xia, LIU Yan, LIANG Shao-shuai, WANG Wen-xiang, WANG Li-juan, ZOU Cong-cong, ZHOU Shun, YOU Feng. 2021.
- 牙鲆gnrh基因的表达分析
- Expression analysis of gnrh in Paralichthys olivaceus
- 海洋科学, 45(6): 118-125
- Marine Sciences, 45(6): 118-125.
- http://dx.doi.org/10.11759/hykx20200824001
-
文章历史
- 收稿日期:2020-08-24
- 修回日期:2020-09-24
2. 中国科学院海洋研究所 实验海洋生物学重点实验室 海洋大科学中心, 山东 青岛 266071;
3. 中国科学院大学, 北京 100049
2. Key Laboratory of Experimental Marine Biology, Center for Ocean Mega-Science, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
促性腺激素释放激素(gonadotropin-releasing hormone, GnRH)是下丘脑-垂体-性腺(hypothalamic pituitary gonadal, HPG)轴的关键上游信号分子。哺乳动物中GnRH通过门静脉作用于垂体[1], 而大多数硬骨鱼中, GnRH由下丘脑合成并以神经分泌的方式释放到垂体, 促进垂体促性腺激素(gonadotrophin, GTH)的合成和释放, 后者可以刺激性腺合成性类固醇激素, 从而调控鱼类性腺分化、发育及生殖等过程[2]。
自20世纪70年代Bmrgus和Matsuo在哺乳动物中发现GnRH以来, 共报道了25种gnrh, 在鱼类中鉴定出19种, 其中17种为鱼类特有[3]。基于系统发育树分析、胚胎起源和脑中神经元的分布, 可将所有gnrh分为gnrh1(sbgnrh)、gnrh2(cgnrh-Ⅱ)和gnrh3 (sgnrh)三种类型[4-5]。一般认为, 在鱼类中, gnrh1具有神经递质活性, 是调节垂体促性腺激素的主要类型, 参与鱼类繁殖相关的功能[2]; gnrh2与生殖行为的调节和摄食行为调节有关[6]; gnrh3除了与性腺分化和发育等繁殖功能相关外, 还与鱼类的感觉功能相关[3]。大多数鱼类的脑中同时表达2种或3种gnrh [7-12]。GnRH与鱼类性腺发育、性成熟及生殖相关的研究有一些报道[13-15], 后来发现gnrh1、gnrh2和gnrh3分别在黑鲷(Acanthopagrus schlegelii)、黄鳝(Monopterus albus)和欧鲈(Dicentrarchus labrax L.)性腺分化期脑和性腺中表达, 在欧鲈中也有明显性别二态性[11-12, 16], 这些研究提示GnRH在一些鱼类的性腺分化中发挥作用。
牙鲆(Paralichthys olivaceus)隶属于鲽形目(Pleuronectiformes)、牙鲆科(Paralichthyidae)、牙鲆属(Paralichthys), 是我国具有重要经济价值的海水养殖鱼种。牙鲆雌性生长显著快于雄性[17], 故研究其性腺分化机制对于进行性别控制, 从而提升其养殖效益有重要价值。研究发现, 其性别决定受遗传和环境等因素影响[18], 对处于性腺分化前的牙鲆, 经17β-雌二醇(E2)处理可使其分化成雌性, 经17α-甲基睾酮(MT)或者高温处理则使其分化为雄性[19-20]。研究还显示, 牙鲆成体脑中表达三种类型的gnrh[21], 但其雌、雄个体组织及性腺分化中的差异表达特征尚未见报道。本文通过实时荧光定量PCR(qPCR)的方法, 分析了三种gnrh在牙鲆雌、雄成体组织, 以及性腺分化前及分化过程中脑和性腺组织中的差异表达, 以期为系统阐述GnRH在鱼类性腺分化和发育中的作用提供参考。
1 材料和方法 1.1 实验用鱼及样品采集牙鲆成鱼全长(total length, TL) (30±5) cm、体重(625±25) g, 购于青岛南山市场, 在中国科学院海洋研究所水族楼鱼缸内暂养3 d用于组织差异表达分析。取样前先用60 mg/L MS-222麻醉。雌雄个体各取3尾, 每尾鱼各取脑、垂体、性腺、胃、脾脏、肠、鳃、肾脏、头肾、肝脏、心脏、眼睛和肌肉组织, 并立即放入液氮中冻存以用于总RNA提取。性腺形状对称, 发育正常, 卵巢呈橘红色, 精巢呈乳白色, 性腺一半用于总RNA提取, 一半用Davison液固定, 通过组织切片HE染色鉴定性腺发育时期[22-23]。
牙鲆异质雌核发育诱导鱼苗在威海圣航科技水产养殖场通过紫外照射和冷休克诱导培育获得[24], 培育至1.2~1.5 cm TL时进行雌雄表型诱导。将鱼苗随机分配到E2、MT和对照3个组中, 每组200尾鱼苗, 分别在3个90 L养殖箱培育, 养殖水温维持在19~21 ℃, 盐度30‰, 溶氧5.8~6.2 mg/L, 每日全量更换过滤海水2~3次, 光周期为14 L: 10 D, 每日投喂3~4次饵料。对照组投喂常规商用饵料。E2和MT组分别投喂含有5 mg/kg的E2和MT (Sigma, 美国)的商用饵料[23], 该饵料配置方法为: E2和MT分别溶解于无水乙醇并均匀喷洒于饵料上, 放置黑暗条件下晾干。培育至鱼苗达到10 cm TL后, 各实验组改投喂常规饵料, 并培育至15 cm TL。在鱼苗2、3、4、6和8 cm TL时, 各组分别随机取3尾鱼, 用40 mg/L MS222麻醉后, 取其脑和性腺组织, 并立即放入液氮中速冻后存放于–80 ℃保存, 用于RNA提取。当鱼苗15 cm TL时, 每组取30尾, 用上述浓度MS222麻醉, 分别采集性腺组织, 用3倍以上体积的Davison液固定, 用于组织切片HE染色分析性比[23]。
1.2 总RNA提取及反转录cDNA采用Trizol(Toroivd, 英国)提取总RNA, 1%琼脂糖凝胶电泳检测其完整性, Nanodrop 2000(Thermo, 美国)检测其浓度及纯度。使用反转录试剂盒HiScript Ⅲ RT SuperMix for qPCR (+gDNA wiper)(诺维赞, 中国南京), 并按其说明书将总RNA反转至cDNA。
1.3 qPCR检测gnrh的表达采用qPCR方法, 检测gnrh1、gnrh2和gnrh3在牙鲆成体组织及性腺分化期脑和性腺中的表达量。根据这些基因的cDNA序列(gnrh1、gnrh2和gnrh3的GenBank编码号分别是DQ074693.1、DQ008580.1和DQ444281.1), 使用Premier 5.0软件设计相应的特异性引物(见表 1)。qPCR反应体系为20 μL, 按照TB GreenTM Premix Ex TaqTM Ⅱ (Tli RNaseH Plus)(宝日医生物, 中国)的说明书配制。其反应程序为: 95 ℃ 30 s预变性; 95 ℃ 5 s, 60 ℃ 30 s, 40个循环。牙鲆β-actin作为内参基因[25]。使用qTOWER3 G荧光定量PCR仪(Jena, 德国)进行扩增, 每个样品重复3次, 利用2–ΔΔCt法计算目的基因相对表达量[26]。使用SPSS软件(IBM SPSS, Armonk, 美国)中的单因素方差分析及Dunnett’s检验, 分析组内和组间基因表达差异的显著性(P < 0.05)。
| 引物名称 | 引物序列(5′-3′) |
| β-actin-qF | GGAATCCACGAGACCACCTACA |
| β-actin-qR | CTGCTTGCTGATCCACATCTGC |
| gnrh1-qF | TCCTGTCAGCACTGGTCCTA |
| gnrh1-qR | CCCTGTCAGTGATGCTTCCA |
| gnrh2-qF | GGAGCTGGACTCTTTTGGCA |
| gnrh2-qR | AGCAGTGCAATGTGGGAAAC |
| gnrh3-qF | GAGCTGGAGGCGACCATTC |
| gnrh3-qR | ATTATGGAACCTTTTCTTTCGGTCG |
性腺组织固定24 h后, 用乙醇和二甲苯梯度脱水处理, 石蜡包埋后切片(厚度5~7 μm), 苏木精和伊红(HE)染色, 乙醇脱水及二甲苯透明, 最后用中性树胶封片。在显微镜(Leica DM LB2, 德国)下观察切片, 判断性别及性腺发育时期[23], 对性腺分化各组样品计算其性比。
2 结果与分析 2.1 gnrh1、gnrh2和gnrh3在牙鲆雌、雄成体组织中的表达用于gnrh1、gnrh2和gnrh3雌、雄成体组织差异表达分析的牙鲆, 经组织学鉴定, 雄性处于精巢Ⅲ期阶段, 雌性处于卵巢Ⅱ期阶段(图 1)。
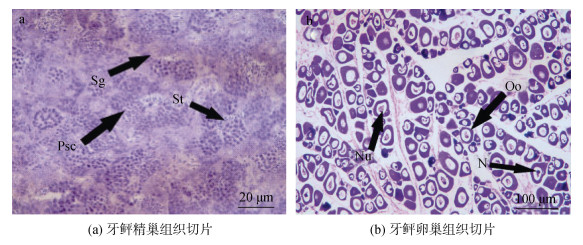 |
| 图 1 牙鲆雌、雄性腺组织切片 Fig. 1 Histological sections of the testis and ovary of Paralichthys olivaceus 注: (a)精巢, 比例尺=20 μm; Sg, 精原细胞; St, 精子细胞; Psc, 初级精母细胞。(b)卵巢, 比例尺=100 μm; Oo, 卵母细胞; N, 细胞核; Nu, 核仁。 |
利用qPCR技术, 对雌、雄成体脑、垂体、性腺、胃、脾脏、肠、鳃、肾脏、头肾、肝脏、心脏、眼睛和肌肉组织中gnrh1、gnrh2和gnrh3表达进行分析。结果表明, gnrh1在雌、雄各组织中几乎都有表达, 且在雌雄个体脑、垂体、肾脏、头肾、肝脏和眼睛中表达较高, 在肾脏中雄性显著高于雌性(P < 0.05); 而精巢中的表达略高于卵巢, 只是没有显著性差异(图 2a)。gnrh2在雌雄肝脏以及雄性的肠、肾脏、头肾、眼睛和肌肉中检测到表达(图 2b)。gnrh3只在雄性的肾脏、眼睛和肌肉中检测到微弱表达(图 2c)。
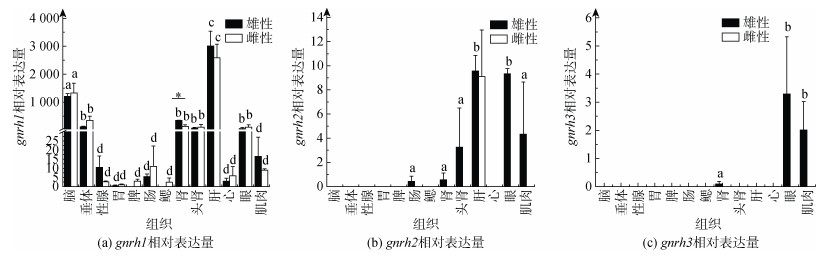 |
| 图 2 gnrh1、gnrh2和gnrh3在牙鲆雌、雄成体组织中的表达 Fig. 2 Expression of gnrh1, gnrh2, and gnrh3 in the male and female adult P. olivaceus tissues 注: n=3; a、b、c、d和a′、b′、c′、d′分别表示雄鱼和雌鱼的不同组织间的显著性(P < 0.05); *表示雌雄个体同组织间的显著性(P < 0.05) |
通过性腺组织切片观察确定性别, 并由此获得各实验组的性比: E2、对照组雌性率均为100%, MT组雄性率为100%。
2.2.1 脑组织中的差异表达qPCR分析结果显示, gnrh1的表达水平随着牙鲆性腺分化的进行呈现上升的趋势, 显示其在性腺分化过程中发挥作用; 在4 cm TL鱼苗中, E2组中的表达显著高于MT组, 也显著高于对照组(P < 0.05) (图 3a), 该结果显示gnrh1的表达在牙鲆卵巢腔出现的时期(4 cm TL)受E2调控。gnrh2表达水平在2 cm TL时, E2组显著高于MT组(P < 0.05), 也显著高于对照组(P < 0.05), 该结果显示gnrh2的表达在牙鲆性腺分化启动时期(2 cm TL)受E2调控; 自3 cm TL, E2和对照组的表达水平均开始出现下降趋势(图 3b), 显示其可能在雌性性腺分化启动时发挥作用。gnrh3在2 cm TL时, E2组和对照组的表达均显著高于MT组(P < 0.05); MT和E2组自6 cm TL开始下降, 三个组均在8 cm TL下降至最低(图 3c), 显示其可能在雌性性腺分化启动和分化中发挥作用, 但在精巢分化启动时作用较小。
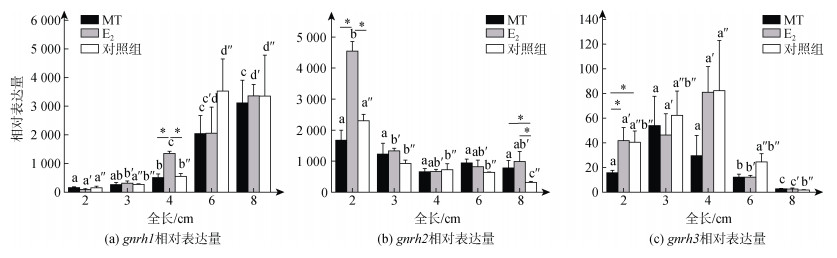 |
| 图 3 gnrh1、gnrh2和gnrh3在牙鲆性腺分化期脑中的表达 Fig. 3 Expression of gnrh1, gnrh2, and gnrh3 in the brain of P. olivaceus during gonadal differentiation period 注: MT, 17α-甲基睾酮组; E2, 17β-雌二醇组; Control, 对照组; n=3; a、b、c, a′、b′、c′, a″、b″、c″、d″分别表示同组不同时期之间的显著性(P < 0.05); *表示同一时期两个组之间的显著性(P < 0.05) |
qPCR结果显示, gnrh1在2 cm TL时, MT、E2和对照组中的表达均为最高水平, 此后在三个组中的表达均下降至较低水平(图 4a)。gnrh2也是在2 cm TL时, 三组中的表达均显著高于后面时期(P < 0.05)(图 4b)。gnrh3在MT和E2组中的表达在2 cm TL时, 显著高于后面时期(P < 0.05)(图 4c)。在各组中gnrh1、gnrh2和gnrh3的表达均呈现随着性腺分化的开始和进行而下降的趋势, 显示出它们在雌性性腺分化启动期在性腺中发挥作用。
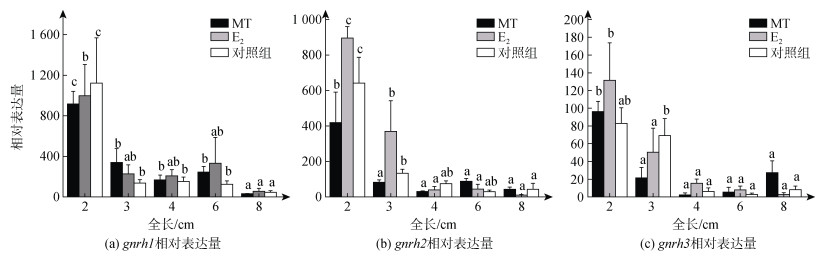 |
| 图 4 gnrh1、gnrh2和gnrh3在牙鲆性腺分化期性腺中的表达 Fig. 4 Expression of gnrh1, gnrh2, and gnrh3 in the gonads of P. olivaceus during gonadal differentiation 注: MT, 17α-甲基睾酮组; E2, 17β-雌二醇组; Control: 对照组; n=3; a、b、c, a′、b′、c′, a″、b″、c″分别表示同组不同时期之间的显著性(P < 0.05) |
在鱼类中, gnrh主要表达于脑和垂体, 在其他组织中也有表达, 但在不同的鱼种中呈现不同的表达模式。在大西洋鳕鱼(Gadus morhua)中, 三种gnrh在组织内的表达较为广泛, 其中gnrh1在卵巢、鳃、心脏、肠道和脾脏中高表达, gnrh2和gnrh3则在脑和卵巢中高表达[27]。在一龄雄性大菱鲆(Schophthalmus maximus)中, gnrh1的表达分布较gnrh2和gnrh3更为广泛, 且在脑、垂体和性腺中呈现高表达的模式, 而gnrh2和gnrh3则只在脑中高表达, 在其他组织中的表达较为微弱[28]。房保海等[1]在性成熟牙鲆中的半定量PCR研究表明, gnrh1在脑、垂体、胃、脾脏、眼睛、肌肉、肠、卵巢和精巢中均表达, gnrh2则在脑、垂体、鳃、头肾和卵巢中有表达。本研究所用牙鲆雌鱼卵巢处于第Ⅱ期, 雄鱼精巢处于第Ⅲ期。定量分析结果显示, gnrh1的表达分布也呈现一个广泛表达的模式, 主要在脑、垂体、肾脏、头肾、肝脏和眼睛中高表达, 卵巢和精巢中则较微弱, 性别差异表达只在肾脏中出现; 其在成体脑中高表达与其在性腺分化期结束时脑中相对较高的表达结果一致。gnrh2和gnrh3均未在成鱼脑、垂体和性腺中检测到表达, 且在其他组织中表达也很微弱, 在脑中未检测到表达的结果与其在性腺分化期结束时脑中较低的表达结果一致。组织表达结果表明, 在卵巢Ⅱ期和精巢Ⅲ期的牙鲆成鱼中, 对性腺发育起作用的主要是gnrh1。在性腺发育其他时期gnrh1, gnrh2和gnrh3在脑和垂体等各组织中的表达特征仍需进一步研究。
性腺分化是鱼类雌雄性别表型形成的必经过程。孙鹏等[23]研究表明, 组织学水平上, 牙鲆在(1.25± 0.13) cm TL时形成原始性腺, 然后在(3.8±0.17) cm TL出现卵巢腔, (6.35±0.34) cm TL出现输精管结构, 分别标志着雌、雄性腺分化的开始, 而到了(8.65± 0.59) cm和(7.6±0.86) cm TL时卵巢和精巢分别形成明显的性腺结构, 标志着性腺分化的基本结束。据此, 本文研究了牙鲆性腺分化启动期(2 cm TL), 性腺分化前期(3 cm TL), 性腺分化期(4~8 cm TL)脑和性腺中gnrh1、gnrh2和gnrh3的表达。在脑中它们呈现不同的表达模式。脑中gnrh1在性腺分化过程中表达量持续上升, gnrh2在2 cm TL时有较高的表达水平, 随后表达水平下降, gnrh3表达水平在4 cm TL前相对较高, 之后下降, 在8 cm TL时基本没有表达。这些结果与军曹鱼(Rachycentron canadum)和鲐鱼(Scomber japonicus)性腺分化期的研究不完全一致, 在这两种鱼中, 雌、雄鱼脑中三种gnrh表达水平均呈上升趋势, 这暗示不同鱼种中三种gnrh对性腺分化的作用可能不同[12, 29]。牙鲆gnrh1在4 cm TL、gnrh2和gnrh3在2 cm TL时, MT和E2组中的表达出现显著差异, 且E2组表达水平均高于MT组, 这些结果提示, 脑中gnrh1与牙鲆性腺分化过程有关, gnrh2和gnrh3可能与性腺分化的启动有关。gnrh调控性别分化的作用在斑马鱼(Danio rerio)中得到了直接证实, gnrh3缺失的斑马鱼在受精后18 d时雄性基因表达显著上升且性腺分化方向向雄性偏移[30]。牙鲆三种gnrh在性腺分化前的原始性腺中表达水平较高, 在性腺分化过程中呈现下降趋势, 这显示性腺中3种gnrh的表达可能都与其分化的启动有关。在雌性先熟的黄鳝性逆转过程中, gnrh2及其剪接体gnrh2-sv表达水平则在初期升高, 并保持较高水平[12], 这说明gnrh2及gnrh2-sv参与了黄鳝性逆转过程中雄性性腺分化的启动, 以及分化过程。有趣的是, 在雄性先熟的黑鲷性逆转过程中, 3种gnrh在性腺中均有表达, 提示黑鲷性腺中gnrh在其性逆转雌性性腺分化过程中发挥作用[31]。因此, gnrh在鱼类的性腺分化中发挥作用, 只是具体作用时间及方式可能存在不同, 有待于进一步的研究和比较。
4 结论综上所述, 牙鲆中gnrh1基因可能参与了其性腺分化的启动、分化过程及其性腺发育的进行, 其gnrh2基因则主要参与了性腺分化的启动, gnrh3基因主要参与了性腺分化的启动和分化过程。这些研究结果对进一步明晰牙鲆性腺分化和发育机制提供了参考。
| [1] |
房保海, 孙修勤, 曲凌云, 等. 牙鲆促性腺激素释放激素基因cGnRH-Ⅱ和sbGnRH的克隆与表达特征分析[J]. 高技术通讯, 2006, 5: 529-533. FANG Baohai, SUN Xiuqin, QU Lingyun, et al. Cloning and expression analysis of gonadotropin-releasing hormone genes cGnRH-Ⅱ and sBGnRH in Japanese flounder[J]. High Technology Communication, 2006, 5: 529-533. DOI:10.3321/j.issn:1002-0470.2006.05.017 |
| [2] |
ZOHAR Y, MUNOZ-CUETO J A, ELIZUR A, KAH O. Neuroendocrinology of reproduction in teleost fish[J]. General and Comparative Endocrinology, 2010, 165: 438-455. DOI:10.1016/j.ygcen.2009.04.017 |
| [3] |
孙静, 孙爱, 吴立新, 等. 鱼类促性腺激素释放激素3(GnRH3)来源、进化及功能研究进展[J]. 农业生物技术学报, 2019, 27(4): 735-742. SUN Jing, SUN Ai, WU Lixin, et al. Research progress on the source, evolution and function of gonadotropin-releasing hormone 3(GnRH3) in fish[J]. Journal of Agricultural Biotechnology, 2019, 27(4): 735-742. |
| [4] |
FEMALD R D, WHITE R B. Gonadotropin-releasing hormone genes: Phylogeny, structure, and functions[J]. Frontiers in Neuroendocrinology, 1999, 20(3): 224-240. DOI:10.1006/frne.1999.0181 |
| [5] |
LETHIMONIER C, MADIGOU T, MUNOZ-CUETO J A, et al. Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish[J]. General and Comparative Endocrinology, 2004, 135: 1-16. DOI:10.1016/j.ygcen.2003.10.007 |
| [6] |
MARVEL M M, SPICER O S, WONG T T, et al. Knockout of Gnrh2 in zebrafish (Danio rerio) reveals its roles in regulating feeding behavior and oocyte quality[J]. General and Comparative Endocrinology, 2019, 280: 15-23. DOI:10.1016/j.ygcen.2019.04.002 |
| [7] |
DUBOIS E A, ZANDBERGEN M A, PEUTE J, et al. Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates[J]. Brain Research Bulletin, 2002, 57: 413-418. DOI:10.1016/S0361-9230(01)00676-1 |
| [8] |
GRAY S L, ADAMS B A, WARBY C M, et al. Transcription and translation of the salmon gonadotropin-releasing hormone genes in brain and gonads of sexually maturing rainbow trout (Oncorhynchus mykiss)[J]. Biology of Reproduction, 2002, 67: 1621-1627. DOI:10.1095/biolreprod.102.004788 |
| [9] |
HILDAH J, SANDVIK G K, EDVARDSEN R B, et al. Identification and gene expression analysis of three GnRH genes in female Atlantic cod during puberty provides insight into GnRH variant gene loss in fish[J]. General and Comparative Endocrinology, 2011, 172: 458-467. DOI:10.1016/j.ygcen.2011.04.010 |
| [10] |
WONG T T, GOTHILF Y, ZMORA N, et al. Developmental expression of three forms of gonadotropin-releasing hormone and ontogeny of the hypothalamic-pituitary-gonadal axis in gilthead seabream (Sparus aurata)[J]. Biology of Reproduction, 2004, 71(3): 1026-1035. DOI:10.1095/biolreprod.104.028019 |
| [11] |
LIN C J, WU G C, DUFOUR S, et al. Activation of the brain-pituitary-gonadotropic axis in the black porgy Acanthopagrus schlegelii during gonadal differentiation and testis development and effect of estradiol treatment[J]. General and Comparative Endocrinology, 2019, 281: 17-29. DOI:10.1016/j.ygcen.2019.05.008 |
| [12] |
FENG K, LUO H R, HOU M X, et al. Alternative splicing of GnRH2 and GnRH2-associated peptide plays roles in gonadal differentiation of the rice field eel, Monopterus albus[J]. General and Comparative Endocrinology, 2018, 267(1): 9-17. |
| [13] |
PARK J W, KIM J H, JIN Y H, et al. Expression profiles of Kiss2, GPR54, and GnRH receptor I mRNAs in the early life stage of Nile tilapia, Oreochromis niloticus[J]. Developmental and Reproductive Biology, 2012, 16: 31-38. |
| [14] |
MOHAMED J S, BENNINGHOFF A D, HOLT G J, et al. Developmental expression of the G protein-coupled receptor 54 and three GnRH mRNAs in the teleost fish cobia[J]. Molecular Endocrinology, 2007, 38: 235-244. DOI:10.1677/jme.1.02182 |
| [15] |
IMANAGA Y, NYUJI M, AMANO M, et al. Characterization of gonadotropin-releasing hormone and gonadotropin in jack mackerel (Trachurus japonicus): Comparative gene expression analysis with respect to reproductive dysfunction in captive and wild fish[J]. Aquaculture, 2014, 428: 226-235. |
| [16] |
MOLES G, CARRILLO M, MANANOS E, et al. Temporal profile of brain and pituitary GnRHs, GnRH-R and gonadotropin mRNA expression and content during early development in European sea bass (Dicentrarchus labrax L.)[J]. General and Comparative Endocrinology, 2007, 150(1): 75-86. DOI:10.1016/j.ygcen.2006.07.012 |
| [17] |
YAMAMOTO E. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck & Schlegel)[J]. Aquaculture, 1999, 173(1): 235-246. |
| [18] |
LUCKENBACH J A, BORSKI R J, DANIELS H V, et al. Sex determination in flatfishes: Mechanisms and environmental influences[J]. Seminars in Cell & Developmental Biology, 2009, 20(3): 256-263. |
| [19] |
YAMAMTO E. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck & Schlegel)[J]. Aquaculture, 1999, 173(1): 235-246. |
| [20] |
SUN P, YOU F, MA D, et al. Sex steroid changes during temperature-induced gonadal differentiation in Paralichthys olivaceus (Temminck & Schegel, 1846)[J]. Journal of Applied Ichthyology, 2013, 29(4): 886-890. DOI:10.1111/jai.12128 |
| [21] |
PHAM X, AMANO M, AMIYA N, et al. Distribution of three GnRHs in the brain and pituitary of the wild Japanese flounder Paralichthys olivaceus[J]. Fisheries science, 2010, 72(1): 89-94. |
| [22] |
FAN Z F, ZOU Y X, JIAO S, et al. Promoter methylation dynamics association with expression of cyp19a and its regulators in Paralichthys olivaceus[J]. Comparative Biochemistry and Physiology, Part A, 2017, 208: 70-79. DOI:10.1016/j.cbpa.2017.02.017 |
| [23] |
孙鹏, 尤锋, 张立敬, 等. 牙鲆性腺分化的组织学研究[J]. 海洋科学, 2009, 33(3): 53-58. SUN Peng, YOU Feng, ZHANG Lijing, et al. Histological study on gonadal differentiation of Japanese flounder[J]. Marine Science, 2009, 33(3): 53-58. |
| [24] |
YOU F, LIU J, WANG X, et al. Study on embryonic development and early growth of triploid and gynogenetic diploid left-eyed flounder, Paralichthys olivaceus[J]. Chinese Journal of Oceanology and Limnology, 2001, 19(2): 147-151. DOI:10.1007/BF02863039 |
| [25] |
ZHENG W, SUN L. Evaluation of housekeeping genes as references for quantitative real time RT-PCR analysis of gene expression in Japanese flounder (Paralichthys olivaceus)[J]. Fish & Shellfish Immunology, 2011, 30(2): 638-645. |
| [26] |
LIVAK K, SCHMITTGEN T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCt Method[J]. Methods, 2000, 25(4): 402-408. |
| [27] |
HILDAHL J, SANDVIK G K, EDVARDSEN R B, et al. Identification and gene expression analysis of three GnRH genes in female Atlantic cod during puberty provides insight into GnRH variant gene loss in fish[J]. General & Comparative Endocrinology, 2011, 172(3): 458-467. |
| [28] |
ZHAO C Y, XU S H, FENG C C, et al. Characterization and differential expression of three GnRH forms during reproductive development in cultured turbot Schophthalmus maximus[J]. Journal of Oceanology and Limnology, 2018(4): 1360-1373. |
| [29] |
SETHU S, HAJIME K, HIROFUMI O, et al. Expression changes of mRNAs encoding kisspeptins and their receptors and gonadotropin-releasing hormones during early development and gonadal sex differentiation periods in the brain of chub mackerel (Scomber japonicus)[J]. General and Comparative Endocrinology, 2015, 222: 20-32. DOI:10.1016/j.ygcen.2014.09.019 |
| [30] |
FENG K, CUI X F, SONG Y L, et al. Gnrh3 regulates PGC proliferation and sex differentiation in developing zebrafish[J]. Endocrinology, 2020, 161(1): 1-12. |
| [31] |
KWANG W A, ERIK R N, HAMID R H, et al. Molecular characterization and expression of three GnRH forms mRNA during gonad sex-change process, and effect of GnRHa on GTH subunits mRNA in the protandrous black porgy (Acanthopagrus schlegeli)[J]. General and Comparative Endocrinology, 2008, 159(1): 38-45. DOI:10.1016/j.ygcen.2008.07.012 |
 2021, Vol. 45
2021, Vol. 45


