文章信息
- 李晓梅, 杨晓琪, 段德麟. 2022.
- LI Xiao-mei, YANG Xiao-qi, DUAN De-lin. 2022.
- 低盐胁迫后两种龙须菜(Gracilariopsis lemaneiformis)叶绿素荧光特征的对比分析
- Effects of low salinity stress on chlorophyll fluorescence characteristics in two Gracilariopsis lemaneiformis strains
- 海洋科学, 46(12): 128-137
- Marine Sciences, 46(12): 128-137.
- http://dx.doi.org/10.11759/hykx20220321002
-
文章历史
- 收稿日期:2022-03-21
- 修回日期:2022-04-17
2. 青岛海洋科学与技术试点国家实验室 海洋生物学与生物技术功能实验室, 山东 青岛 266237;
3. 中国科学院大学, 北京 100049
2. Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China
龙须菜(Gracilariopsis lemaneiformis)隶属红藻门(Rhodophyta)江蓠目(Gracilariales)龙须菜属(Gracilariopsis), 是一种生长在潮间带的大型经济红藻[1]。龙须菜富含红藻多糖、膳食纤维、维生素等物质, 具有降血压和调节血脂的功效, 因而是健康海洋食品的来源[2-4]。龙须菜是琼胶提取的重要原料[5-6], 近20年来已在我国南、北方实现了规模化养殖。2015年, 以龙须菜/江蓠属为原料制备的琼胶产量达到114 100 t(干质量), 约占世界琼胶产量的91%[2, 7]。龙须菜还可用作鲍养殖的活鲜饵料[3]。与许多大型海藻一样, 龙须菜养殖对“碳汇渔业”发挥重要贡献[8]。目前在中国, 龙须菜养殖产量仅次于海带, 已成为第二大海藻养殖对象[9]。
温度、盐度、营养盐、光照等是影响龙须菜生长的重要因素。高温能够诱发龙须菜氧化应激, 造成龙须菜细胞损伤, 严重时甚至会导致藻体死亡[10]。Kang等[11]认为龙须菜在高浓度CO2和NH4+培养, 可提高光合效率, 增加生长速率。强光可促进龙须菜的光合作用[12]。在低盐和高盐条件下, 龙须菜光合放氧速率显著下降且呼吸速率明显提高, 从而导致生长速率降低[13]。
盐度是影响海藻生长的最重要因素之一, 盐度会影响藻类的生理活性, 从而影响其生长[1, 14-15]。受潮间带环境的影响, 龙须菜需要经历周期性的低潮失水变化, 此时暴雨天气会使藻体面临低盐胁迫。生长在低盐条件下的龙须菜叶绿素含量降低, 类胡萝卜素含量升高, 以提高其在低盐条件下的光保护能力[16]。Chang等[17]发现低盐能促进龙须菜琼胶产量积累以维持细胞形态。蔡西栗等[18]发现低盐胁迫后龙须菜细胞色素体结板层结构明显肿胀且细胞间孔状联系部分受到破坏, 细胞内红藻淀粉颗粒变少。目前, 低盐如何影响龙须菜光合作用的研究未见报道。
本研究以龙须菜981和其绿色突变型为对象, 通过对比其叶绿素荧光特征, 探讨低盐条件(盐度28和22)下光合生理响应机制差异, 可为龙须菜响应低盐胁迫应答提供理论依据, 也可为低盐环境下龙须菜养殖品种的选择提供参考。
1 材料与方法 1.1 材料采集实验所用龙须菜981(编号Wt)和其绿色突变型(编号Gr)均采自山东高绿水产养殖有限公司(122.6°E, 37.2°N)。龙须菜绿色突变型, 是龙须菜981品种自然突变后连续无性繁殖而获得, 其颜色表型稳定(图 1)。从养殖筏架采集上述2种龙须菜, 低温条件下运回实验室。用海水清洗, 去除其表面附着物, 分别在过滤的自然海水(盐度为32), 于培养箱(GXZ-280A, 宁波江南仪器厂)中预培养5 d, 培养条件: 温度20 ℃, 光强72 μmol photons·m−2·s−1, 光周期L∶D=12 h∶12 h。
 |
| 图 1 龙须菜981和龙须菜绿色突变型 Fig. 1 Strain 981 and the G. lemaneiformis green mutant |
使用光学折射盐度计(SYY1-1, 济南光学仪器厂)测定自然海水盐度为32, 用去离子水将海水稀释至盐度28(低盐条件1)和22(低盐条件2)2种低盐条件。将预培养的龙须菜981和龙须菜绿色突变型置于盐度28和22两种低盐条件进行胁迫处理, 处理过程中温度始终保持20 ℃, 低盐胁迫过程中的光强和光周期与预培养条件的一致。采集低盐胁迫处理前(即第0天)以及低盐胁迫后第1天、第2天和第3天的龙须菜样品, 用于后续的叶绿素荧光生理分析。
1.2.2 快速光响应曲线(RLC)的测量与分析挑选生长状态良好的龙须菜981和龙须菜绿色突变型藻体(5~10 cm), 使用Closed FluorCam FC800荧光成像系统(Photon Systems Instruments Ltd., Brno, Czech Republic)检测二者快速光响应曲线, 光合有效辐射(PAR)梯度依次为: 0、66.29、169.86、377.00、584.14、791.28、998.42 μmol photons·m-2·s-1, 持续时间为10 s, 测定相对电子传递速率(relative electron transport rate, rETR), 采用Origin 2018软件, 参照Eilers和Peeters[19]方法, 拟合快速光响应曲线得到光能利用效率(α)、最大相对电子传递速率(rETRmax)和半饱和光强(Ik)。
1.2.3 快速叶绿素荧光诱导动力学曲线(OJIP)检测采用Closed FluorCam FC800荧光成像系统(Photon Systems Instruments Ltd., Brno, Czech Republic), 对两种龙须菜的叶绿素荧光诱导动力学曲线进行检测, 测量前暗适应15 min。其中20 μs(FO)、2 ms(FJ)、30 ms(FI)和1 000 ms(FP)分别记录了O、J、I和P各相的荧光强度, 饱和光强度为3 000 μmol photons·m-2·s-1。从获得的OJIP曲线各相荧光强度进行JIP-test分析[20], 所得参数如表 1。
| 荧光参数 | 定义 |
| FO = F20 μs | 初始荧光 |
| FK = F300 μs | K相荧光 |
| FJ = F2 ms | J相荧光 |
| FI = F30 ms | I相荧光 |
| Fm = FP | 最大荧光 |
| FV= Fm−FO | 可变荧光 |
| FV/Fm | PSII反应中心处于完全开放状态的量子产量 |
| VJ = (FJ−FO)/(Fm−FO) | J点相对可变荧光 |
| VI = (FI−FO)/(Fm−FO) | I点相对可变荧光 |
| VK= (FK−FO)/(Fm−FO) | K点相对可变荧光 |
| φEO = FV/Fm × (1−VJ) | PSII反应中心处于完全开放状态的用于电子传递的量子产额 |
| MO = 4(FK−FO)/(Fm−FO) | 归一化荧光上升起点处的斜率 |
| ABS/RC = (MO/VJ)/(1/FV/Fm) | PSII反应中心处于完全开放状态的单位反应中心的光吸收 |
| PIabs = (RC/ABS) × [FV/Fm/(1−FV/Fm)] × [(1−VJ)/VJ] | 以吸收光能为基础的性能指数 |
采用Closed FluorCam FC800荧光成像系统(Photon Systems Instruments Ltd., Brno, Czech Republic), 对2种龙须菜的NPQ进行测量, 测量前暗适应15 min。给予待测样品饱和光(光强3 000 μmol photons·m-2·s-1)照射后继续使用远红外光照射, 得到NPQ值, 其反映待测样品的热耗散水平。
1.2.5 数据分析所有数据均以平均数±标准差(Mean±SD)表示。使用SPSS(21.0)进行单因素方差分析, P < 0.05为显著差异。使用Origin 2018和Excel软件绘制图形。
Eilers和Peeters[19]建立了浮游植物光合作用速率与光强关系的动态模型, 该模型通过最小二乘法对快速光响应曲线进行拟合, 拟合公式如下, 公式中的a、b和c是通过模型拟合得到的常数参数。
| $ r_{\mathrm{ETR}}=\frac{P_{\mathrm{AR}}}{a \times P_{\mathrm{AR}}{ }^2+b \times P_{\mathrm{AR}}+c} . $ | (1) |
各参数的计算公式分别是:
| $ \alpha {\text{ = }}\frac{1}{c}, $ | (2) |
| $ r_{\text {ETR max }}=\frac{1}{b+2 \sqrt{a \times c}} , $ | (3) |
| $ I_{\mathrm{k}}=\frac{c}{b+2 \sqrt{a \times c}} . $ | (4) |
JIP-test是快速叶绿素荧光诱导动力学分析的数学模型, 是专门用来评估毫秒级或微秒级叶绿体氧化还原级联反应的生物物理工具[21-22]。JIP-test可将瞬时荧光变化转化为参数的定量变化, 其代表每一个被检测的捕光复合体的总能量流入和流出之间的平衡, 并提供关于吸收能量的可能分配的信息, 利用这些方程, 可以描述PSII复合体之间的能量通信[23]。
2 结果 2.1 龙须菜981和龙须菜绿色突变型叶绿素荧光对比分析对正常生长状态下的龙须菜981和龙须菜绿色突变型的OJIP瞬时曲线检测分析, 我们发现龙须菜绿色突变型的O、J、I和P相的荧光强度均高于龙须菜981(图 2a)。JIP-test分析表明, 龙须菜绿色突变型的PIabs高于龙须菜981(图 2b, P < 0.01), 说明龙须菜绿色突变型的PSII整体光合性能要高于龙须菜981。
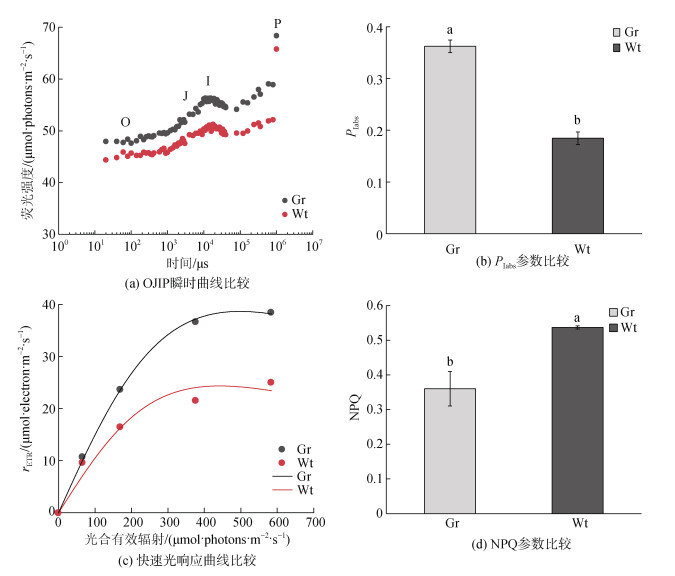 |
| 图 2 龙须菜981和龙须菜绿色突变型的叶绿素荧光特征比较 Fig. 2 Comparison of chlorophyll fluorescence in strain 981 and the G. lemaneiformis green mutant |
另外我们发现, 相同光化光梯度下, 龙须菜绿色突变型的rETR整体高于龙须菜981(图 2c)。指数模型拟合快速光响应曲线(表 2)发现, 龙须菜绿色突变型的α、rETRmax和Ik值均高于龙须菜981, 这表明龙须菜绿色突变型的光合效率和半饱和光强均高于龙须菜981。
| 参数 | Gr | Wt |
| α | 0.160±0.008 | 0.131±0.001 |
| rETRmax | 38.0±0.8 | 24.1±0.6 |
| Ik | 238±9 | 199±8 |
NPQ分析结果表明(图 2d), 龙须菜绿色突变型的NPQ显著低于龙须菜981(P < 0.05), 说明龙须菜绿色突变型的热耗散水平低于龙须菜981。
2.2 低盐胁迫对龙须菜981和龙须菜绿色突变型荧光参数的影响低盐28处理3 d后, 龙须菜绿色突变型FV/Fm有微弱下降, 幅度为3.6%, 而随着胁迫时间的延长, 龙须菜981的FV/Fm的下降趋势增大(图 3a), 下调幅度可达10.2%。在盐度28下, 龙须菜绿色突变型的φEO无显著变化(图 3b), 龙须菜981则下降了9.5%。龙须菜绿色突变型的FV/Fm和φEO受盐度28影响较小, 而龙须菜981的FV/Fm和φEO则略有下降。
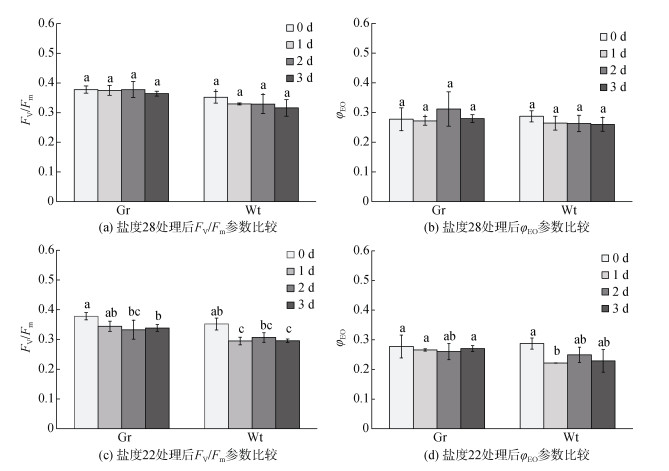 |
| 图 3 低盐胁迫3 d内2种龙须菜叶绿素荧光参数对比 Fig. 3 Comparison of the chlorophyll fluorescence parameters in strain 981 and the G. lemaneiformis green mutant exposed to low salinity stress for three days |
低盐22处理后, 随着时间的延长, 龙须菜绿色突变型的FV/Fm下降趋势增加, 在第3天出现显著下降(图 3c), 幅度可达10.4%; 而龙须菜981的FV/Fm也有明显下降趋势, 幅度可达16.0%。在盐度22下, 龙须菜绿色突变型的φEO下降2.5%, 而龙须菜981第1天出现显著下降(图 3d), 第3天后下降幅度为20.3%。这表明低盐22条件胁迫处理后, 龙须菜981的FV/Fm和φEO下降幅度均高于龙须菜绿色突变型。
低盐胁迫处理3 d后, 两种龙须菜rETR均有下调趋势(图 4a, b)。低盐28条件胁迫3 d后, 龙须菜绿色突变型α下降了9.7%, 而龙须菜981则下降了14.5%(图 4c); 龙须菜绿色突变型rETRmax下降了2.5%, 而龙须菜981发生显著下降(图 4d), 下降幅度为20.4%。低盐22条件胁迫3 d后, 龙须菜绿色突变型和龙须菜981的α均出现下降, 幅度分别为12.1%和31.5%, rETRmax也分别下降了16.3%和22.1%。在盐度28和22两种低盐条件下, 龙须菜绿色突变型光响应曲线参数α和rETRmax下降幅度均小于龙须菜981。
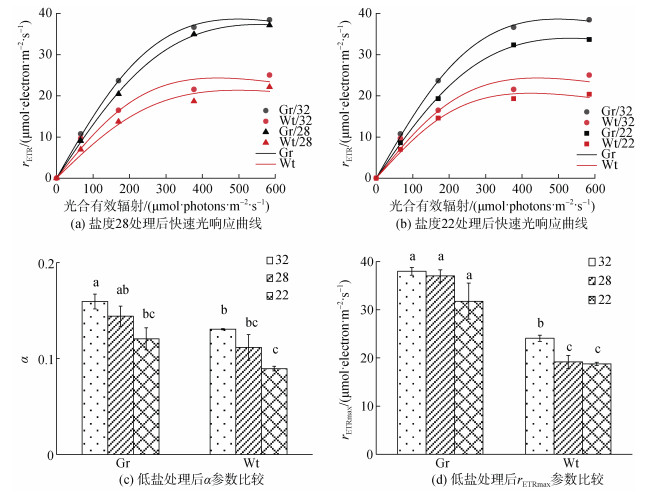 |
| 图 4 低盐胁迫3 d后两种龙须菜快速光响应曲线及参数比较 Fig. 4 Comparison of the rapid light curve in strain 981 and the G. lemaneiformis green mutant exposed to low salinity stress for three days |
龙须菜绿色突变型是龙须菜981品种自然突变后经连续无性繁殖得到的表型稳定的突变体, 其颜色的改变, 可能与藻胆蛋白的组成相关[4, 24-25], 而藻胆蛋白是藻类光合作用重要的辅助色素, 因此我们推测龙须菜绿色突变型光合性能与龙须菜981品种存在较大差异。
植物中, PSII是光合作用电子传递链中响应外界环境变化最敏感的组分[26]。快速叶绿素荧光诱导动力学曲线OJIP, 主要反映了PSII的原初光化学反应及光合结构电子传递状态等过程的变化[27]。在检测的两种龙须菜的OJIP曲线中, 龙须菜绿色突变型O、J、I和P相荧光强度均高于龙须菜981, 这说明其PSII反应中心和电子传递效率要高于龙须菜981。PIabs是以吸收光能为基础的性能指数, 能够准确反映藻类光合结构的状态[28]。本研究中, 龙须菜绿色突变型的PIabs值高于龙须菜981, 说明其光合性能要优于龙须菜981。
快速光响应曲线RLC提供了关于藻类电子传递速率的信息, 同时反映光合结构对瞬时及长期光照的响应, 已被用于海藻一系列生理生态研究[29]。对比分析龙须菜981和龙须菜绿色突变型的快速光响应曲线, 拟合分析后发现龙须菜绿色突变型有较高的α值, α反映藻体光化学反应的启动速率, 因此龙须菜绿色突变型光能利用效率要高于龙须菜981。最大相对电子传递速率rETRmax反映了藻类吸收光能中用于电子链传递的份额, 代表了光合速率的最大潜力, 决定了光合作用的能力[30]。相比于龙须菜981, 龙须菜绿色突变型中呈现较高的rETRmax值, 表明其潜在的最大光合作用能力大于龙须菜981。Ik反映了藻类光合作用对强光的耐受能力, 龙须菜绿色突变型Ik高于龙须菜981, 表明其对强光的耐受能力高于龙须菜981。
综上来看, 相比较龙须菜981, 龙须菜绿色突变型龙须菜对强光耐受能力较强, 光合性能显著上调, 具体表现为: 捕光能力提升、PSII整体性能增强以及电子传递效率提高。龙须菜绿色突变型光合性能的增强可能有助于提升其固碳能力[31-32], 这有利于其通过光合作用进行物质积累增加产量。
3.2 低盐胁迫后龙须菜981与龙须菜绿色突变型叶绿素荧光对比分析盐度是决定海藻的生长和繁殖的重要环境因子, 因潮汐、暴雨等自然过程带来的海水盐度波动会干扰海藻的生理活性[33-34]。在低盐条件下Gracilaria parvispora最大光合速率Pmax显著降低[35]。智利江蓠幼孢子体和配子体在低盐条件生长受抑制[36]。低盐会抑制帚状江蓠细胞分裂, 从而影响其在低盐环境中的生长[15]。低盐胁迫影响缢江蓠光合速率和蛋白质合成效率, 从而抑制其生长过程中色素、藻胆蛋白以及可溶性蛋白的积累, 同时活性氧积累, 致使细胞膜发生脂质过氧化, 导致细胞膜流动性下降[37]。Kumar等[38]发现极端低盐处理后Gracilaria corticata活性氧的形成超过了临界限度, 并伴随着抗氧化酶活性下降, 对其造成严重威胁。
先前有报道称, 低盐胁迫下龙须菜生长速率受显著抑制[18]。基于快速光响应曲线的拟合分析发现, 低盐28条件处理后龙须菜981的rETRmax显著下调, 并且随着盐度降低至22, rETRmax下调幅度增大, 表明低盐处理对龙须菜981的光能转化和电子传递产生了负面影响[39-40]。Li等[41]发现低盐胁迫后红藻光能捕获及利用受抑制与负责光能转化传递至光系统反应中心的藻胆蛋白、类胡萝卜素和叶绿素等光合色素含量降低有关。然而, 龙须菜绿色突变型在盐度22和28低盐处理后rETRmax变化不显著, 表明其光能转化和电子传递能力受低盐干扰较少, 暗示其具有较高的低盐耐受性。
作为PSII在胁迫下效率状态的指示性参数, FV/Fm代表PSII最大光化学效率, 反映所有PSII反应中心处于完全开放状态的量子产量, 广泛用于评价环境对海藻光合结构的影响[42-44], 可有效评估光系统反应中心的状态。低盐22处理后, 龙须菜981的FV/Fm的下降程度加剧, 幅度高于龙须菜绿色突变型, 说明盐度22降低了PSII原初光能转换效率, 使龙须菜981的PSII潜在活性中心受损, 导致最大光量子产量下降。同时, 龙须菜981的φEO下降明显, 表明盐度22抑制了龙须菜981光合作用电子传递量子产额, 影响了PSII受体侧QA、QB和PQ等位点的电子传递, 这与先前的研究相一致[45-46]。
综上来看, 盐度28对龙须菜981光能转化和电子传递产生了负面影响, 对龙须菜绿色突变型光合性能影响微弱。盐度22抑制了龙须菜981对光能捕获及利用, 其PSII反应中心显著受抑制, PSII受体侧光合电子传递受阻, 从而严重降低了龙须菜981的光合能力。相对于龙须菜981, 盐度22对龙须菜绿色突变型光能捕获及利用、PSII反应中心和电子传递的抑制程度较轻, 表明龙须菜绿色突变型对低盐耐受性较高。
4 结论本研究结果表明低盐胁迫能影响龙须菜的光合活性。相对龙须菜981, 龙须菜绿色突变型的光合性能提升, 其光合性能对低盐胁迫的耐受性要高于龙须菜981。因此, 龙须菜绿色突变型可作为龙须菜后续种质改良的理想资源。
| [1] |
ZHOU W, SUI Z H, WANG J G, et al. An orthogonal design for optimization of growth conditions for all life history stages of Gracilariopsis lemaneiformis (Rhodophyta)[J]. Aquaculture, 2013, 392: 98-105. |
| [2] |
隋正红, 胡依依, 周伟, 等. 龙须菜栽培与遗传育种[J]. 中国海洋大学学报(自然科学版), 2020, 50(9): 98-104. SUI Zhenghong, HU Yiyi, ZHOU Wei, et al. Review on the cultivation and genetic breeding of Gracilariopsis lemaneiformis (Rhodophyta)[J]. Periodical of Ocean University of China, 2020, 50(9): 98-104. DOI:10.16441/j.cnki.hdxb.20200026 |
| [3] |
ZHANG X Q, HU C Y, SUN X, et al. Comparative tran—scriptome analysis reveals chitooligosaccharidesinduced stress tolerance of Gracilariopsis lemaneiformis under high temperature stress[J]. Aquaculture, 2020, 519: 734876. DOI:10.1016/j.aquaculture.2019.734876 |
| [4] |
HUANG X Y, ZANG X N, WU F, et al. Transcriptome sequencing of Gracilariopsis lemaneiformis to analyze the genes related to optically active phycoerythrin synthesis[J]. PloS One, 2017, 12(1): e0170855. DOI:10.1371/journal.pone.0170855 |
| [5] |
LIU Y T, SUN H Y, DING Y, et al. A novel heat shock protein from Gracilariopsis lemaneiformis: gene cloning and transcription analysis in response to heat stress[J]. Journal of Applied Phycology, 2019, 30(6): 3623-3631. |
| [6] |
CRAIGIE J S, WEN Z C, VANDERMEER J P. Interspecific, intraspecific and nutritionally-determined variations in the composition of agars from Gracilaria spp.[J]. Botanica Marina, 1984, 27(2): 55-61. |
| [7] |
PORSE H, RUDOLPH B. The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook[J]. Journal of Applied Phycology, 2017, 29(5): 2187-2200. DOI:10.1007/s10811-017-1144-0 |
| [8] |
GAO G, GAO L, JIANG M J, et al. The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication[J]. Environmental Research Letters, 2022, 17(1): 014018. DOI:10.1088/1748-9326/ac3fd9 |
| [9] |
农业农村部渔业渔政管理局. 2020中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2020. Bureau of Fisheries, Ministry of Agriculture and Rural Affairs. China fishery statistics yearbook[M]. Beijing: China Agriculture Press, 2020. |
| [10] |
FU F, SUI Z H, ZHOU W, et al. UV-irradiation mutation of tetraspores of Gracilariopsis lemaneiformis and screening of thermotolerant strains[J]. Journal of Applied Phycology, 2014, 26(1): 647-656. DOI:10.1007/s10811-013-0087-3 |
| [11] |
KANG J W, KAMBEY C, SHEN Z, et al. The shortterm effects of elevated CO2 and ammonium concentrations on physiological responses in Gracilariopsis lemaneiformis (Rhodophyta)[J]. Fisheries and Aquatic Sciences, 2017, 20(3): 1-8. |
| [12] |
WU H Y, JIANG H, LIU C X, et al. Growth, pigment composition, chlorophyll fluorescence and antioxidant defenses in the red alga Gracilaria lemaneiformis (Gracilariales, Rhodophyta) under light stress[J]. South African Journal of Botany, 2015, 100: 27-32. DOI:10.1016/j.sajb.2015.05.017 |
| [13] |
陈月, 蔡西栗, 孙雪, 等. 龙须菜在不同盐度胁迫下的琼胶积累及相关生理生化变化的研究[J]. 水生生物学报, 2020, 44(6): 1322-1329. CHEN Yue, CAI Xili, SUN Xue, et al. Agar accumulation and physiology and biochemistry changes of Gracilariopsis lemaneiformis under different salinity stresses[J]. Acta Hydrobiologica Sinica, 2020, 44(6): 1322-1329. |
| [14] |
JELIANI Z Z, YOUSEFZADI M, POUR J S, et al. Growth, phytochemicals, and optimal timing of planting Gracilariopsis persica: an economic red seaweed[J]. Journal of Applied Phycology, 2018, 30(1): 525-533. DOI:10.1007/s10811-017-1217-0 |
| [15] |
YU C H, LIM P E, PHANG S M. Effects of irradiance and salinity on the growth of carpospore-derived tetrasporophytes of Gracilaria edulis and Gracilaria tenuistipitata var liui (Rhodophyta)[J]. Journal of Applied Phycology, 2013, 25(3): 787-794. DOI:10.1007/s10811-012-9960-8 |
| [16] |
JIANG H, ZOU D H, CHEN B B. Effects of reduced carbon supply and sunlight on photosynthetic and antioxidant activities of Gracilariopsis lemaneiformis, and subsequent changes of these activities under recovery conditions with different salinities[J]. Aquaculture, 2018, 493: 258-263. DOI:10.1016/j.aquaculture.2018.05.014 |
| [17] |
CHANG L P, SUI Z H, FU F, et al. Relationship between gene expression of UDP-glucose pyrophosphorylase and agar yield in Gracilariopsis lemaneiformis (Rhodophyta)[J]. Journal of Applied Phycology, 2014, 26(6): 2435-2441. DOI:10.1007/s10811-014-0277-7 |
| [18] |
蔡西栗, 孙雪, 邵旻玮, 等. 龙须菜对不同盐度胁迫的应激生理响应[C]//中国水产学会. 2011年中国水产学会学术年会论文摘要集. 2011-11-15, 福建厦门, 2011: 33. CAI Xili, SUN Xue, SHAO Minwei, et al. The physiological response of marine red algae Gracilaria lemaneiformis to different salinity stress[C]//Collection of abstracts of the 2011 Annual Conference of Chinese Fishery Society, 2011-11-15, Xiamen, China, 2011: 33. |
| [19] |
EILERS P H C, PEETERS J C H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton[J]. Ecological Modelling, 1988, 42(3/4): 199-215. |
| [20] |
ZUSHI K, KAJIWARA S, MATSUZOE N. Chlorophyll a fluorescence OJIP transient as a tool to characterize and evaluate response to heat and chilling stress in tomato leaf and fruit[J]. Scientia Horticulturae, 2013, 148: 39-46. |
| [21] |
STRASSER R J, SRIVASTAVA A, TSIMILLI-MICHAEL M. The fluorescence transient as a tool to characterize and screen photosynthetic samples[M]// YUNUS M, PATHRE U, MOHANTY P. Probing photosynthesis: mechanisms, regulation and adaptation. Taylor and Francis, London, UK, 2000: 445-483.
|
| [22] |
STRASSER B J, STRASSER R J. Measuring fast fluorescence transients to address environmental questions: the JIP-test[M]. Photosynthesis: from Light To Biosphere. Kluwer Academic Publishers, The Netherlands, 1995, 5: 977-980.
|
| [23] |
STRASSER R J, TSIMILLI-MICHAEL M, SRIVASTAVA A. Analysis of the chlorophyll fluorescence transient[M]//PAPAGEORGIOU E, GOVINDJEE G C. Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, 2004, 19: 321-362.
|
| [24] |
张学成, 程晓杰, 施定基. 江蓠属藻胆蛋白的研究-II. 龙须菜藻胆蛋白光谱特性和荧光动力学研究[J]. 海洋学报, 1999, 21(1): 90-96. ZHANG Xuecheng, CHENG Xiaojie, SHI Dingji. Studies on phycobiliprotein in Gracilaria−II. Characterization on absorption and emission spectra and fluorescent dynamics in intact thallus of Gracilaria lemaneiformis[J]. Acta Oceanologica Sinica, 1999, 21(1): 90-96. |
| [25] |
张学成, 张锦东, 隋正红, 等. 江蓠属藻胆蛋白的研究-I. 诱变、突变体筛选及藻胆蛋白的光谱特性[J]. 青岛海洋大学学报, 1996, 26(3): 318-326. ZHANG Xuecheng, ZHANG Jindong, SUI Zhenghong, et al. Studies on phycobiliproteins from Gracilaria−I. Mutagenesis, mutation selection and characterization of absorption spectra and florescent emission spectra[J]. Journal of Ocean University of Qingdao, 1996, 26(3): 318-326. |
| [26] |
JARVI D, SUORSA M, ARO E M. PhotosystemII repair in plant chloroplasts−Regulation, assisting proteins and shared components with photosystem II biogenesis[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2015, 1847(9): 900-909. DOI:10.1016/j.bbabio.2015.01.006 |
| [27] |
STIRBET A, GOVINDJEE. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient[J]. Journal of Photochemistry and Photobiology B-Biology, 2011, 104(1/2): 236-257. |
| [28] |
BUCHER S F, BERNHARDT-ROMERMANN M, ROMERMANN C. Chlorophyll fluorescence and gas exchange measurements in field research: an ecological case study[J]. Photosynthetica, 2018, 56(4): 1161-1170. DOI:10.1007/s11099-018-0809-5 |
| [29] |
RALPH P J, GADEMANN R. Rapid light curves: A powerful tool to assess photosynthetic activity[J]. Aquatic Botany, 2005, 82(3): 222-237. DOI:10.1016/j.aquabot.2005.02.006 |
| [30] |
杨小舟, 郑新庆, 林荣澄, 等. 三种大型绿藻光合能力的差异及其在珊瑚养殖中的应用[J]. 生态学杂志, 2014, 33(6): 1528-1533. YANG Xiaozhou, ZHENG Xinqing, LIN Rongcheng, et al. Photosynthetic capacity of three common species of macroalgae and the application in coral aquarium[J]. Chinese Journal of Ecology, 2014, 33(6): 1528-1533. DOI:10.13292/j.1000-4890.20140327.006 |
| [31] |
GEEL C, VERSLUIS W, SNEL J F H. Estimation of oxygen evolution by marine phytoplankton from measurement of the efficiency of photosystem II electron flow[J]. Photosynthesis Research, 1997, 51: 61-70. DOI:10.1023/A:1005779112140 |
| [32] |
GILBERT M, WILHELM C, RICHTER M. Bio-optical modelling of oxygen evolution using in vivo fluorescence: Comparison of measuredand calculated photosynthesis/irradiance (P-I) curves in four representative phytoplankton species[J]. Journal of Plant Physiology, 2000, 157: 307-314. DOI:10.1016/S0176-1617(00)80052-8 |
| [33] |
GUO H, YAO J T, SUN Z M, et al. Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera[J]. Chinese Journal of Oceanology and Limnology, 2015, 33(2): 410-418. DOI:10.1007/s00343-015-4105-y |
| [34] |
MACLER B A. Salinity effects on photosynthesis, carbon allocation, and nitrogen assimilation in the red alga, Gelidium coulteri[J]. Plant Physiology, 1988, 88(3): 690-694. DOI:10.1104/pp.88.3.690 |
| [35] |
ORDUNA-ROJAS J, GARCIA-RODRIGUEZ L D, LOPEZ-MEYER M, et al. Photosynthetic and respiratory responses of Gracilaria parvispora from the southeastern Gulf of California[J]. Journal of Applied Phycology, 2013, 25(6): 1855-1861. |
| [36] |
GUILLEMIN M L, SEPULVEDA R D, CORREA J A, et al. Differential ecological responses to environmental stress in the life history phases of the isomorphic red alga Gracilaria chilensis (Rhodophyta)[J]. Journal of Applied Phycology, 2013, 25(1): 215-224. DOI:10.1007/s10811-012-9855-8 |
| [37] |
王艳平, 谢恩义, 黄翔鹄, 等. 缢江蓠生长率及其生化组分含量分析[J]. 南方农业学报, 2017, 48(6): 1099-1105. WANG Yanping, XIE Enyi, HUANG Xianghu, et al. Growth rate of Gracilaria salicornia and its biochemical composition contents[J]. Journal of Southern Agriculture, 2017, 48(6): 1099-1105. DOI:10.3969/j.issn.2095-1191.2017.06.26 |
| [38] |
KUMAR M, KUMARI P, GUPTA V, et al. Biochemical responses of red alga Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress[J]. Journal of Experimental Marine Biology and Ecology, 2010, 391(1): 27-34. |
| [39] |
STAMENKOVIC M, STEINWALL E, WULFF A. Cultivation and photophysiological characteristics of desmids in moderately saline aquaculture wastewater[J]. Journal of Phycology, 2021, 57(3): 726-741. |
| [40] |
DONG L L, ZHANG G Q, LI W, et al. Early toxic effects of Cd2+ on photosynthetic activity of six freshwater algae species[J]. Polish Journal of Environmental Studies, 2020, 29(3): 2159-2166. DOI:10.15244/pjoes/110519 |
| [41] |
LI Y F, LIU J G, ZHANG L T, et al. Changes of photosynthetic performances in mature thalli of the red alga Gelidium amansii (Gelidiaceae) exposed to different salinities[J]. Marine Biology Research, 2016, 12(6): 631-639. DOI:10.1080/17451000.2016.1177654 |
| [42] |
LIU C X, ZOU D H, YANG Y F, et al. Temperature responses of pigment contents, chlorophyll fluorescence characteristics, and antioxidant defenses in Gracilariopsis lemaneiformis (Gracilariales, Rhodophyta) under different CO2 levels[J]. Journal of Applied Phycology, 2017, 29(2): 983-991. DOI:10.1007/s10811-016-0971-8 |
| [43] |
HAO B B, WU H P, YOU J Q, et al. Biomass and physiological responses of green algae and diatoms to nutrient availability differ between the presence and absence of macrophytes[J]. Ecological Indicators, 2021, 129: 107987. DOI:10.1016/j.ecolind.2021.107987 |
| [44] |
TAN L J, XU W J, HE X L, et al. The feasibility of FV/Fm on judging nutrient limitation of marine algae through indoor simulation and insitu experiment[J]. Estuarine, Coastal and Shelf Science, 2019, 229: 106411. DOI:10.1016/j.ecss.2019.106411 |
| [45] |
LI Y, PANG T, LIU J, et al. Light absorption and impacts of low salinities on photosynthetic behaviour in the epiphytic alga Neosiphonia savatieri (Rhodomelaceae, Rhodophyta)[J]. Journal of Applied Phycology, 2017, 29(3): 1673-1681. |
| [46] |
SATOH K, SMITH C M, FORK D C. Effects of salinity onprimary processes of photosynthesis in the red alga Porphyra perforata[J]. Plant Physiology, 1983, 73(3): 643-647. DOI:10.1104/pp.73.3.643 |
 2022, Vol. 46
2022, Vol. 46


