文章信息
- 刘雅萍, 方圣涛, 时振振, 季乃云. 2022.
- LIU Ya-ping, FANG Sheng-tao, SHI Zhen-zhen, JI Nai-yun. 2022.
- 深海冷泉草酸青霉Penicillium oxalicum 13-37的化学成分研究
- Chemical constituents of Penicillium oxalicum 13-37 from cold-seep sediments
- 海洋科学, 46(5): 115-121
- Marine Sciences, 46(5): 115-121.
- http://dx.doi.org/10.11759/hykx20210414002
-
文章历史
- 收稿日期:2021-04-14
- 修回日期:2022-01-19
2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
深海是全球最大的独立生态系统,蕴含着丰富的微生物资源[1-2],其独特极端的生存环境使得深海微生物具备与陆地和浅海微生物不同的代谢途径及遗传背景[3-5]。深海沉积物是深海微生物的重要来源,近些年来,科学家们从深海中分离培养出大量的微生物并对其次生代谢产物进行研究[6-8]。其中深海沉积物来源微生物次生代谢产物也愈发受到关注,现已得到多种类型的化合物,如酮类、甾体类、萜类、二酮哌嗪类、生物碱类、脂类、苯腙类、喹唑啉类和过氧化物等[9-12],但对深海冷泉沉积物来源微生物次生代谢产物研究较少。因此,研究深海冷泉沉积物来源微生物的次生代谢产物,是丰富海洋天然产物结构多样性的重要途径,并且对于进一步开发利用深海资源具有重要意义。
根据前期对深海冷泉沉积物来源草酸青霉的研究[12],本文对其次生代谢产物进行进一步分离纯化,得到了11个化合物(1-11),包括1个xanthone酮类化合物及其开环衍生物(1和2)、7个蒽醌类化合物(3-9)、2个萜类化合物(10和11)。综合运用现代波谱技术包括核磁共振、质谱、红外光谱、X-射线单晶衍射及与参考文献数据对比等方法对其结构进行鉴定,并对其中新化合物(1)的生物活性进行了测试。化合物1-11结构见图 1。
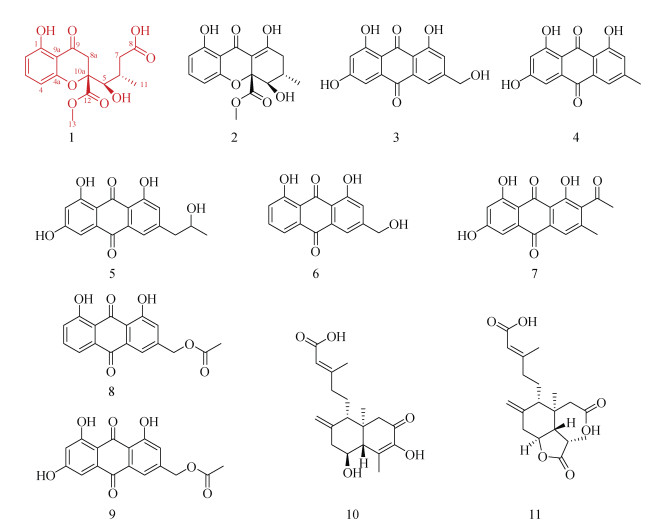 |
| 图 1 化合物1-11的结构 Fig. 1 Structures of compounds 1-11 |
供试卤虫(Artemia salina),为实验室保存卵孵化。供试微藻: 赤潮异弯藻(Heterosigma akashiwo)、东海原甲藻(Prorocentrum donghaiense)和海洋卡盾藻(Chattonella marina),为实验室培植继代微藻。供试菌株: 细菌包括柠檬假单胞菌(Pseudoalteromonas citrea)、大肠杆菌(Escherichia coli)、金黄色葡萄球菌(Staphylococcus aureus)、鳗弧菌(Vibrio anguillarum)、哈氏弧菌(V. harveyi)和副溶血弧菌(V. parahemolyticus)和灿烂弧菌(V. splendidus),真菌包括灰葡萄孢菌(Botrytis cinerea)、黄瓜枯萎病菌(Fusarium oxysporum f. sp. cucumerium)、西瓜枯萎病菌(F. oxysporum f. niveum)和芦笋茎枯病菌(Phomopsis asparagi),为实验室保存菌株。
石油醚(分析纯)、乙酸乙酯(分析纯)、二氯甲烷(分析纯)、甲醇(分析纯)、乙腈(色谱纯),上海国药集团化学试剂有限公司。
Bruker Avance III 500型超导核磁共振波谱仪,Bruker公司; 超高效液相色谱-质谱联用仪,Waters公司; Nicolet iS50红外光谱仪Thermo Fisher Scientific公司,Agilent 1260型高效液相色谱系统(Eclipese SB-C18色谱柱,9.4 mm × 250 mm,5 μm),Agilent技术有限公司。
1.2 实验方法 1.2.1 菌株的分离与培养菌株分离于南海深海冷泉沉积物,经ITS基因测序,鉴定该菌株为Penicillium oxalicum,菌种(CGMCC No.19913)保存在中国微生物菌种保藏管理委员会普通微生物中心。
采用大米培养基将菌株进行批量发酵, 得到草酸青霉代谢产物粗提物536 g[12]。
1.2.2 单体化合物的分离与纯化将粗提物经硅胶柱层析,依次用一定比例的石油醚-乙酸乙酯和二氯甲烷-甲醇进行梯度洗脱,合并相同或类似部分得到8个组分(1-8)[12]。
组分4 (石油醚-乙酸乙酯,2∶1)经反相硅胶柱层析(甲醇-水,7∶3),Sephadex LH-20 (甲醇)凝胶柱层析和制备薄层层析得到化合物8 (3.1 mg)。
组分5 (石油醚-乙酸乙酯,1∶1)经反相硅胶柱层析(甲醇-水,17∶3-9∶1),得到组分5-1 (甲醇-水,17∶3)和5-2 (甲醇-水,9∶1),5-1经硅胶柱层析(石油醚-乙酸乙酯,1∶1)和半制备高效液相色谱(乙腈-水,3∶2-7∶3,0.1%冰醋酸)得化合物9 (4.8 mg)和化合物7 (2.1 mg); 5-2经硅胶柱层析(石油醚-乙酸乙酯,2: 1)得化合物4 (36.7 mg)。
组分6 (乙酸乙酯)经反相硅胶柱层析(甲醇-水,13∶7-9∶1),得到组分6-1 (甲醇-水,13∶7),6-2 (甲醇-水,7∶3),6-3 (甲醇-水,3∶1),6-4 (甲醇-水,9∶1)。6-1经Sephadex LH-20 (甲醇)凝胶柱层析再经过硅胶柱层析(二氯甲烷-甲醇,30∶1)得到化合物10 (13.2 mg); 6-2经Sephadex LH-20 (甲醇)凝胶柱层析分别得化合物11 (8.8 mg)和化合物3 (6.9 mg); 6-3经Sephadex LH-20 (甲醇)凝胶柱层析、半制备高效液相色谱(乙腈-水,1∶4-2∶3)分别得化合物1 (4.7 mg)和化合物2 (5.6 mg); 6-4经硅胶柱层析(石油醚-乙酸乙酯,2∶1-1∶1),分别得化合物5 (8.9 mg)和化合物6 (22.0 mg)。
1.2.3 化合物结构鉴定综合运用核磁共振、红外光谱、质谱、X-射线单晶衍射及与文献数据比较等方法,对所得化合物进行结构鉴定和解析。
1.2.4 化合物生物活性测试参照文献所用的实验方法,测定新化合物对卤虫的毒性以及抗微藻、抗细菌、抗真菌活性[13-15]。
2 实验结果 2.1 化合物的结构鉴定化合物1: 白色粉末。[α]28 D −72 (c 0.056, MeOH); UV (MeOH) λmax (log ε) 271 (3.6) nm; IR (KBr) vmax 3 476, 2 955, 1 732, 1 648, 1 628, 1 580, 1 462, 1 359, 1 290, 1 228, 1 049, 795 cm–1; 高分辨质谱HRESIMS (m/z [M + Na]+ 361.090 7,计算为: 361.089 4,提示分子式为: C16H18O8,不饱和度为8。1H NMR谱(DMSO-d6)显示该化合物含有3个芳香区信号: δH 7.47 (1H, dd, J = 8.0, 7.1 Hz, H-3), δH 6.53 (1H, d, J = 7.1 Hz, H-2)和δH 6.48 (1H, d, J = 8.0 Hz, H-4),2个甲基信号: δH 3.60 (3H, s, H-13)和δH 0.91 (3H, d, J = 6.6 Hz, H-11)。进一步分析13C NMR和DEPT NMR谱(DMSO-d6),发现该化合物分子中有16个碳原子,分别为7个季碳信号: δC 196.7 (C-9, C), δC 172.5 (C-8, C), δC 170.4 (C-12, C), δC 160.8 (C-1, C), δC 159.7 (C-4a, C), δC 107.2 (C-9a, C), δC 87.5 (C-10a, C), 5个次甲基信号: δC 107.7 (C-2, CH), δC 138.7 (C-3, CH), δC 109.2 (C-4, CH), δC 74.7 (C-5 CH)和δC 30.9 (C-6, CH),2个亚甲基信号: δC 40.5 (C-8a, CH2)和δC 39.5 (C-7, CH2)及2个甲基信号: δC 52.9 (C-13, CH3)和δC 13.9 (C-11, CH3)。化合物1在分离过程中可以由化合物2逐渐变化得到,其核磁谱图也显示出与blennolide B[16]的相似之处,通过仔细比较核磁数据,发现化合物1与blennolide B的不同之处在于,blennolide B化合物C-8a位的季碳消失,变成一个亚甲基,再根据化合物1的C-8处化学位移推测C-8位连接了一个羧基,因此推测该化合物1在C-8a和C-8之间开环,是blennolide B的开环衍生物。通过HMBC谱图中,H-7与C-8的相关确定羧基与C-7相连,结合COSY谱中H-6与H-5、H-7和H-11的相关,将骨架由C-5扩展到C-8,再通过COSY、HMBC相关(图 2)推出化合物1的平面结构。化合物2的旋光度为[α]20 D+102 (c 0.18, MeOH),与blennolide B的文献报道基本一致,确定化合物2的绝对构型为为5R,6S和10aR,因此,确定该新化合物的绝对构型为5R,6S和10aR,是一个新的xanthone酮类开环衍生物,命名为seco-blennolide B。核磁数据如下: δH 11.48 (1H, brs, OH-1), 7.47 (1H, dd, J = 8.0, 7.1 Hz, H-3), 6.53 (1H, d, J = 7.1 Hz, H-2), 6.48 (1H, d, J = 8.0 Hz, H-4), 5.72 (1H, d, J = 6.3 Hz, OH-5), 3.84 (1H, dd, J = 6.3, 2.2 Hz, H-5), 3.60 (3H, s, H-13), 3.42 (1H, d, J = 17.1 Hz, H-8aa), 3.00 (1H, d, J = 17.1 Hz, H-8ab), 2.49 (1H, dd, J = 15.8, 7.0 Hz, H-7a), 2.28 (1H, dd, J = 15.8, 7.0 Hz, H-7b), 2.22 (2H, m, H-6), 0.91 (3H, d, J = 6.6 Hz, H-11); 13C NMR (DMSO-d6, 125 MHz): δC 196.7 (C-9, C), 172.5 (C-8, C), 170.4 (C-12, C), 160.8 (C-1, C), 159.7 (C-4a, C), 107.7 (C-2, CH), 107.2 (C-9a, C), 138.7 (C-3, CH), 109.2 (C-4, CH), 87.5 (C-10a, C), 74.7 (C-5 CH), 52.9 (C-13, CH3), 40.5 (C-8a, CH2), 39.5 (C-7, CH2), 30.9 (C-6, CH), 13.9 (C-11, CH3)。
 |
| 图 2 化合物1重要的COSY和HMBC相关 Fig. 2 Key COSY and HMBC correlations of compound 1 |
化合物2: C16H16O7,淡黄色无定型粉末。核磁波谱数据如下: δH 13.90 (1H, brs, OH-8), 11.29 (1H, s, OH-1), 7.43 (1H, t, J = 8.2 Hz, H-3), 6.54 (2H, dd, J = 8.2, 0.8 Hz, H-4), 6.47 (1H, dd, J = 8.2, 0.8 Hz, H-2), 5.95 (1H, d, J = 5.5 Hz, OH-5), 3.77 (1H, m, H-5), 3.56 (3H, s, H-13), 2.58 (1H, m, H-7b), 2.40 (2H, m, H-6), 2.28 (1H, m, H-7a), 1.01 (3H, d, J = 6.4 Hz, H-11); 13C NMR (DMSO-d6, 125 MHz): δC 185.7 (C-9, C), 179.6 (C-8, C), 170.7 (C-12, C), 161.4 (C-1, C), 160.0 (C-4a, C), 138.4 (C-3, CH), 110.0 (C-2, CH), 108.2 (C-4, CH), 107.2 (C-9a, C), 102.2 (C-8a, C), 85.6 (C-10a, C), 75.8 (C-5, CH), 53.1 (C-13, CH3), 36.3 (C-7, CH2), 30.3 (C-6, CH), 18.2 (C-11, CH3)。经过文献检索与数据比对发现化合物2与文献报道的已知化合物blennolide B[16]波谱数据基本一致,因此将化合物2鉴定为blennolide B。
化合物3: C15H10O6,橙黄色粉末状固体。1H NMR数据(DMSO-d6, 500 MHz): δH 12.07 (1H, s, OH-8), 12.04 (1H, s, OH-1), 7.61 (1H, s, H-5), 7.22 (1H, s, H-7), 7.10 (1H, d, J = 2.4 Hz, H-4), 6.57 (1H, d, J = 2.4 Hz, H-2), 5.57(1H, brs, OH-6), 4.60 (2H, s, H-11); 13C NMR (DMSO-d6, 125 MHz): δC 190.2 (C-9, C), 181.8 (C-10, C), 166.1 (C-3, C), 164.9 (C-1, C), 161.9 (C-8, C), 153.3 (C-6, C), 135.6 (C-10a, C), 133.4 (C-4a, C), 121.2 (C-7, CH), 117.5 (C-5, CH), 114.5 (C-8a, C), 109.4 (C-4, CH), 109.3 (C-9a, C), 108.3 (C-2, CH), 62.5 (C-11, CH2)。经过文献检索与数据比对发现化合物3与文献报道的已知化合物citreorosein[17]数据基本一致,因此将化合物3鉴定为citreorosein。
化合物4: C15H10O5,黄色粉末状固体。核磁波谱数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 12.09 (1H, brs, 8-OH), 12.03 (1H, brs, OH-1),7.49 (1H, d, J = 1.2 Hz, H-5), 7.17 (1H, m, H-4), 7.12 (1H, d, J = 2.4 Hz, H-7), 6.60 (1H, d, J = 2.4 Hz, H-2), 2.42 (3H, s, H-11); 13C NMR (DMSO-d6, 125 MHz): δC 189.7 (C-9, C), 181.4 (C-10, C), 165.6 (C-1, C), 164.5 (C-3, C), 161.4 (C-8, C), 148.3 (C-6, C), 135.1 (C-4a, C), 132.9 (C-10a, C), 124.2 (C-7, CH), 120.5 (C-5, CH), 113.4 (C-8a, C), 109.0 (C-9a, C), 108.8 (C-4, CH), 108.0 (C-2, CH), 21.5 (C-11, CH3)。经过文献检索与数据比对发现化合物4与文献报道的已知化合物emodin[18]数据基本一致,因此将化合物4鉴定为emodin。
化合物5: C17H14O6,黄色粉末状固体。核磁波谱数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 12.11 (1H, s, OH-8), 12.02 (1H, s, OH-1), 11.39 (1H, s, OH-6), 7.55 (1H, s, H-2), 7.20 (1H, s, H-4), 7.14 (1H, d, J = 2.4 Hz, H-5), 6.61 (1H, d, J = 2.4 Hz, H-7), 4.69 (1H, m, H-2’), 3.91 (1H, m, OH-4), 2.73 (2H, m, H-1’), 1.11 (3H, d, J = 6.1 Hz, H-3’); 13C NMR (DMSO-d6, 125 MHz): δC 190.3 (C-9, C), 182.0 (C-10, C), 166.0 (C-8, C), 164.9 (C-6, C), 161.6 (C-1, C), 150.6 (C-3, C), 135.7 (C-5a, C), 133.0 (C-10a, C), 125.3 (C-2, CH), 121.5 (C-4, CH), 114.1 (C-1a, C), 109.5 (C-9a, CH), 109.2 (C-5, C), 108.4 (C-7, CH), 67.0 (C-2’, CH), 45.5 (C-1’, CH2), 23.9 (C-3’, CH3)。经文献比对,化合物5的核磁数据与isorhodoptilometrin[19]基本一致,因此将化合物5鉴定为isorhodoptilometrin。
化合物6: C15H10O5,黄色粉末状固体。核磁波谱数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 11.99 (1H, brs, OH-8), 11.93 (1H, brs, OH-1), 7.84 (1H, t, J = 7.7 Hz, H-6), 7.74 (1H, d, J = 7.5 Hz, H-5), 7.72 (1H, s, H-4), 7.40 (1H, d, J = 8.3 Hz, H-7), 7.31 (1H, s, H-2), 4.66 (2H, s, H-11); 13C NMR (DMSO-d6, 125 MHz): δC 192.2 (C-9, C), 182.0 (C-10, C), 162.1 (C-3, C), 161.8 (C-1, C), 154.2 (C-8, C), 137.8 (C-6, CH), 133.9 (C-10a, C), 133.6 (C-4a, C), 124.9 (C-7, CH), 121.2 (C-5, CH), 119.8 (C-8a, C), 117.6 (C-4, CH), 116.4 (C-9a, C), 115.0 (C-2, CH), 62.5 (C-11, CH2)。经文献比对,化合物6的波谱数据与已知化合物aloe-emodin[20]的文献报道数据基本一致,因此将化合物6鉴定为aloe-emodin。
化合物7: C17H12O6,淡黄色粉末状固体。核磁数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 11.98 (1H, s, OH-1), 7.53 (1H, s, H-4), 7.10 (1H, s, H-5), 6.56 (1H, s, H-7), 2.55 (3H, s, H-12), 2.31 (3H, s, H-13); 13C NMR (DMSO-d6, 125 MHz): δC 203.6 (C-11, C), 189.4 (C-9, C), 181.6 (C-10, C), 165.1 (C-8, C), 158.2 (C-1, C), 143.7 (C-3, C), 136.5 (C-2, C), 135.5 (C-4a, C), 133.1 (C-10a, C), 121.5 (C-4, CH), 114.5 (C-9a, C), 110.2 (C-5, CH), 109.0 (C-8a, C), 109.0 (C-6, C), 108.5 (C-7, CH), 32.1 (C-12, CH3), 19.8 (C-13, CH3)。经文献检索对比,该化合物的核磁数据与2-acetylemodin[21]一致,因此将化合物7鉴定为2-acetylemodin。
化合物8: C17H12O6,黄色粉末状固体。核磁波谱数据如下: 1H NMR数据(CDCl3, 500 MHz): δH 12.08 (1H, s, OH-8), 12.05 (1H, s, OH-1), 7.80 (1H, d, J = 7.8 Hz, H-5), 7.77 (1H, brs, H-4), 7.68 (1H, t, J = 7.7 Hz, H-6), 7.29 (1H, d, J = 8.0 Hz, H-7), 7.24 (1H, s, H-2), 5.14 (2H, s, H-11), 2.18 (3H, s, CH3CO); 13C NMR (CDCl3, 125 MHz): δC 192.3 (C-9, C), 181.1 (C-10, C), 170.2 (C-2’, C=O), 162.4 (C-1, C), 162.1 (C-8, C), 146.4 (C-3, CH), 136.9 (C-6, C), 133.7 (C-10a, C), 133.4 (C-4a, C), 124.6 (C-7, CH), 122.3 (C-2, CH), 120.1 (C-5, CH), 118.4 (C-4, CH), 115.8 (C-8a, C), 115.2 (C-9a, C), 64.7 (C-11, CH2), 20.7 (C-1’, CH3)。经文献对比,化合物8的核磁数据与11-O-acetylaloeemodin[22]基本一致,因此将该化合物鉴定为11-O-acetylaloeemodin。
化合物9: C17H12O7,淡黄色粉末状固体。核磁波谱数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 12.13 (1H, brs, OH-1), 12.13 (1H, brs, OH-9), 7.62 (1H, s, H-4), 7.30 (1H, s, H-2), 7.08 (1H, s, H-6), 6.51 (1H, s, H-8), 5.18 (2H, s, H-11), 2.15 (3H, s, CH3CO); 13C NMR (DMSO-d6, 125 MHz): δC 189.4 (C-10, C), 181.7 (C-5, C), 170.6 (C-12, C), 167.6 (C-7, C), 165.2 (C-9, C), 161.8 (C-1, C), 145.9 (C-3, C), 135.5 (C-5a, C), 133.8 (C-4a, C), 122.7 (C-2, CH), 118.2 (C-4, CH), 115.6 (C-10a, C), 110.3 (C-6, CH), 108.9 (C-9a, C), 108.4 (C-8, CH), 64.7 (C-11, CH2), 21.1 (C-13, CH3)。经文献对比,化合物9的核磁数据与phaseolorin I[23]基本一致,因此将该化合物鉴定为phaseolorin I。
化合物10: C19H26O5,无色晶体; 核磁数据如下: 1H NMR数据(CD3OD, 500 MHz): δH 5.65 (1H, brs, H-14), 5.07 (1H, brs, H-17a), 4.72 (1H, brs, H-17b), 3.74 (1H, ddd, J = 10.7, 10.5, 5.5 Hz, H-5), 2.77 (1H, dd, J = 12.5, 5.5 Hz, H-7a), 2.63 (1H, d, J = 16.8 Hz, H-1a), 2.59 (1H, dq, J = 10.7, 1.9 Hz, H-5), 2.36 (1H, d, J = 16.8 Hz, H-1b), 2.35 (1H, m, H-12a), 2.31 (1H, dd, J = 12.5, 5.5 Hz, H-7b), 2.15 (3H, d, J = 1.2 Hz, H-16), 2.10 (3H, d, J = 1.9 Hz, H-18), 2.09 (1H, m, H-12b), 2.05 (1H, brd, J = 10.8 Hz, H-9), 1.62 (1H, m, H-11), 0.71 (3H, s, H-19); 13C NMR (CD3OD, 125 MHz): δC 194.5 (C-2, C), 170.2 (C-15, C), 161.2 (C-13, C), 146.4 (C-3, C), 145.0 (C-8, C), 134.7 (C-4, C), 117.1 (C-15, CH), 110.0 (C-17, CH2), 70.2 (C-6, CH), 55.0 (C-5, CH), 53.0 (C-9, CH), 51.5 (C-1a, CH2), 48.8 (C-7, CH2), 45.0 (C-10, C), 40.3 (C-12, CH2), 23.0 (C-11, CH2), 18.8 (C-16, CH3), 15.3 (C-18, CH3), 14.0 (C-19, CH3)。13C NMR和DEPT谱(CD3OD)显示该化合物共19个碳原子,包括3个甲基信号,5个亚甲基信号,4个次甲基和7个季碳信号,结合1H NMR谱(CD3OD)信息,推断该化合物应为降二萜类化合物,其绝对构型通过单晶数据(CCDC 2024004)确定: M = 334.40, a = 16.035 7 (3) Å, b = 16.035 7 (3) Å, c = 24.684 1 (5) Å, α = 90°, β = 90°, γ = 120°, V = 5497.0 (2) Å3, T = 100.(2) K, 该单晶属于P63空间群, Z = 12, μ(Cu Kα) = 0.708 mm–1, 测试衍射点数: 74578,其中不相关衍射点数: 7259 (Rint = 0.0552)。最终得到R1: 0.0302 (I > 2σ(I)), wR(F2): 0.0842 (I > 2σ(I)), R1: 0.0303 (all data), wR(F2): 0.0844 (all data), F2的GOF值: 1.061, Flack常数: 0.02 (4),单晶衍射图如下(图 3)。经文献对比,化合物10与化合物penitholabene[24]结构一致,因此将其鉴定为penitholabene。
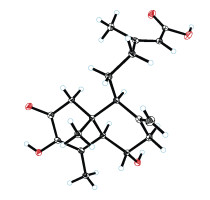 |
| 图 3 化合物10的单晶结构图 Fig. 3 X-ray crystallographic structure of compound 10 |
化合物11: C18H24O6,白色粉末状固体。核磁波谱数据如下: 1H NMR数据(DMSO-d6, 500 MHz): δH 5.57 (1H, s, H-13), 5.19 (1H, s, H-16a), 4.84 (1H, s, H-16b), 4.38 (1H, m, H-8), 2.89 (1H, m, H-7a), 2.77 (1H, m, H-3), 2.48 (1H, d, J = 14.2, H-18a), 2.41 (1H, dd, J = 11.5, 7.2 Hz, H-9), 2.35 (1H, d, J = 14.2 Hz, H-18b), 2.18 (1H, m, H-11a), 2.16 (1H, m, H-7b), 2.09 (3H, d, J = 1.2 Hz, H-15), 2.01 (1H, m, H-5), 1.89 (1H, m, H-11b), 1.71 (1H, m, H-10a), 1.51 (1H, m, H-10b), 1.18 (3H, d, J = 7.7 Hz, H-17), 0.78 (3H, s, H-20); 13C NMR (DMSO-d6, 125 MHz): δC 179.3 (C-2, C), 172.9 (C-19, C), 167.9 (C-14, C), 159.4 (C-12, C), 142.6 (C-6, C), 116.4 (C-13, CH), 113.9 (C-16, CH2), 76.9 (C-8, CH), 52.8 (C-9, CH), 50.8 (C-5, CH), 42.6 (C-7, CH2), 42.4 (C-18, CH2), 40.1 (C-4, C), 39.6 (C-11, CH2), 38.7 (C-3, CH), 22.7 (C-10, CH2), 18.9 (C-15, CH3), 17.8 (C-20, CH3), 12.2 (C-17, CH3)。经与文献对比,化合物11与化合物paecilomycine A[25]核磁数据基本一致,因此将其鉴定为paecilomycine A。
2.2 活性结果新化合物(100 μg/mL)对卤虫的致死率为11.9%,对东海原甲藻和海洋卡盾藻的抑制率分别为85.3%和43.2%,该浓度下对赤潮异弯藻无明显的活性。新化合物(20 μg/片)对副溶血弧菌的纸片抑菌圈直径为9.3 mm,该浓度下对其他测试细菌、真菌无明显活性。
3 结论本文从深海冷泉采集的沉积物样品中分离得到一株海洋真菌草酸青霉,对其进行批量发酵培养,所得粗提物利用硅胶柱层析、Sephadex LH-20凝胶柱层析、反相硅胶柱层析、制备薄层层析和半制备高效液相色谱等分离手段得到1个新的单体化合物seco-blennolide B (1),其他化合物通过1H NMR、13C NMR等方式鉴定为blennolide B (2)、citreorosein (3)、emodine (4)、aloe-emodin (5)、isorhodoptilometrin (6)、11-O-acetylaloeemodin (8)、phaseolorin I(9)、2-acetylemodin (7)、penitholabene (10)和paecilomycine A (11)。化合物1为新的xanthone酮类开环衍生物,化合物10和11为首次从深海冷泉草酸青霉中分离到的已知萜类化合物。此外,本文首次获取化合物10的单晶结构,并对其单晶数据进行报道。活性测试结果表明,化合物1对海洋卡盾藻、东海原甲藻的生长具有一定的抑制作用,对卤虫表现出微弱的毒性,对副溶血弧菌表现出弱的抑制作用,对其他测试细菌和真菌、微藻无明显活性。
| [1] |
王风平, 周悦恒, 张新旭, 等. 深海微生物多样性[J]. 生物多样性, 2013, 21(4): 445-455. WANG Fengping, ZHOU Yueheng, ZHANG Xinxu, et al. Biodiversity of deep-sea microorganisms[J]. Biodiversity Science, 2013, 21(4): 445-455. |
| [2] |
张宇, 肖湘. 深海微生物的研究与开发[J]. 生命科学, 2012, 24(9): 986-990. ZHANG Yu, XIAO Xiang. Research and development on deep sea microbiology[J]. Chinese Bulletin of Life Sciences, 2012, 24(9): 986-990. |
| [3] |
马新华, 郑卫敏, 周芳, 等. 2株深海来源真菌次级代谢产物的研究[J]. 中国海洋药物, 2018, 37(5): 41-46. MA Xinhua, ZHENG Weimin, ZHOU Fang, et al. Studies on the secondary metabolites of two deep-sea-derived fungi[J]. Chinese Journal of Marine Drugs, 2018, 37(5): 41-46. |
| [4] |
席峰, 郑天凌, 焦念志, 等. 深海微生物多样性形成机制浅析[J]. 地球科学进展, 2004, 19(1): 38-46. XI Feng, ZHENG Tianling, JIAO Nianzhi, et al. a preliminary analysis of mechanism of deep sea microorganisms diversity[J]. Advance in Earth Sciences, 2004, 19(1): 38-46. DOI:10.3321/j.issn:1001-8166.2004.01.006 |
| [5] |
蔡松岩, 何建林, 白锴凯, 等. 深海真菌次级代谢产物及其生物活性研究进展[J]. 国际药学研究杂志, 2020, 47(12): 1033-1046. CAI Songyan, HE Jianlin, BAI Kaikai, et al. Secondary metabolites of deep-sea fungi and their biological activities: research advances[J]. International Journal of Pharmaceutical Research, 2020, 47(12): 1033-1046. |
| [6] |
曾奇, 仲伟茂, 向瑶, 等. 南海深海沉积物中52株真菌的初步分离鉴定及其代谢产物活性[J]. 微生物学通报, 2018, 45(9): 1904-1915. ZENG Qi, ZHONG Weimao, XIANG Yao, et al. Isolation, identification and evaluation of 52 fungi from the deep-sea sediments of South China Sea[J]. Microbiology China, 2018, 45(9): 1904-1915. |
| [7] |
曾奇, 仲伟茂, 王发左. 深海来源真菌次级代谢产物研究进展[J]. 天然产物研究与开发, 2018, 30(3): 501-514. ZENG Qi, ZHONG Weimao, WANG Fazuo. Secondary metabolites isolated from deep-sea-derived fungi[J]. Natural Product Research and Development, 2018, 30(3): 501-514. |
| [8] |
李梦瀛, 闫培生, 高秀君, 等. 深海真菌多样性及其代谢产物生物活性的研究进展[J]. 生物技术进展, 2015, 5(3): 170-175. LI Mengying, YAN Peisheng, GAO Xiujun, et al. Progress on diversity and secondary metabolites biological activity of deep-sea fungi[J]. Current Biotechnology, 2015, 5(3): 170-175. DOI:10.3969/j.issn.2095-2341.2015.03.04 |
| [9] |
WANG Y N, MENG L H, WANG B G. Progress in research on bioactive secondary metabolites from deep-sea derived microorganisms[J]. Marine Drugs, 2020, 18(12): 614. DOI:10.3390/md18120614 |
| [10] |
CHI L P, LI X M, WAN Y P, et al. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512[J]. Journal of Natural Products, 2020, 83(12): 3652-3660. DOI:10.1021/acs.jnatprod.0c00860 |
| [11] |
LV F Y, LI X M, CHI L P, et al. A new acyclic peroxide from Aspergillus nidulans SD-531, a fungus obtained from deep-sea sediment of cold spring in the South China Sea[J]. Journal of Oceanology and Limnology, 2020, 38(4): 1225-1232. DOI:10.1007/s00343-020-0052-3 |
| [12] |
LIU Y P, FANG S T, SHI Z Z, et al. Phenylhydrazone and quinazoline derivatives from the cold-seep-derived fungus Penicillium oxalicum[J]. Marine Drugs, 2021, 19(1): 9. |
| [13] |
SHI Z Z, FANG S T, MIAO F P, et al. Trichocarotins A-H and trichocadinin A, nine sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma virens[J]. Bioorganic Chemistry, 2018, 81: 319-325. DOI:10.1016/j.bioorg.2018.08.027 |
| [14] |
SONG Y P, SHI Z Z, YIN X L, et al. Tricholumin A, a highly transformed ergosterol derivative from the algaendophytic fungus Trichoderma asperellum[J]. Organic Letters, 2018, 6306-6309. |
| [15] |
SHI Z Z, LIU X H, LI X N, et al. Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2[J]. Journal of Agricultural and Food Chemistry, 2020, 68(52): 15440-15448. DOI:10.1021/acs.jafc.0c05586 |
| [16] |
QIN T, JOHNSON R P, PORCO J A. Vinylogous addition of siloxyfurans to benzopyryliums: a concise approach to the tetrahydroxanthone natural products[J]. Journal of the American Chemical society, 2011, 133(6): 1714-1717. DOI:10.1021/ja110698n |
| [17] |
MONDAL A, SAHA N, RAJPUT A, et al. Chemoenzymatic reduction of citreorosein and its implications on aloe-emodin and rugulosin C (bio)synthesis[J]. Organic & Biomolecular Chemistry, 2019, 17: 8711-8715. |
| [18] |
SAID G, HOU X M, LIU X, et al. Antimicrobial and cytotoxic activities of secondary metabolites from the soft coral derived fungus Aspergillus sp.[J]. Chemistry of Natural Compounds, 2019, 55(3): 531-533. DOI:10.1007/s10600-019-02732-5 |
| [19] |
ZHANG Y H, HOU X M, YU M L, et al. Secondary metabolites and their bioactivities from the gorgonianderived fungus Aspergillus versicolor[J]. Chemistry of Natural Compounds, 2019, 55(2): 327-330. DOI:10.1007/s10600-019-02680-0 |
| [20] |
SHI D H, HUANG W, LI C, et al. Design, synthesis and molecular modeling of aloe-emodin derivatives as potent xanthine oxidase inhibitors[J]. European Journal of Medicinal Chemistry, 2014, 75: 289-296. DOI:10.1016/j.ejmech.2014.01.058 |
| [21] |
CAMERON D W, GROSSLEY M J, FEUTRILL G I, et al. Chemistry of the Coccoidea. V. Synthesis of 2-acetylemodin and of deoxyerythrolaccin[J]. Cheminform, 1978, 31: 1363-1370. |
| [22] |
付文卫, 谭昌恒, 孟祥雪, 等. 美味猕猴桃根中化学成分的分离与鉴定[J]. 中国药物化学杂志, 2010, 20(2): 116-118. FU Wenwei, TAN Changheng, MENG Xiangxue, et al. Isolation and identification of the chemical constituents from root of Actinidia deliciosa[J]. Chinese Journal of Medicinal Chemistry, 2010, 20(2): 116-118. |
| [23] |
NIU Z, CHEN Y, GUO H, et al. Cytotoxic polyketides from a deep-sea sediment derived fungus Diaporthe phaseolorum FS431[J]. Molecules, 2019, 24(17): 3062. DOI:10.3390/molecules24173062 |
| [24] |
LI Y L, LIU W, HAN S Y, et al. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea- derived fungus Penicillium thomii YPGA3[J]. Fitoterapia, 2020, 146: 104691. DOI:10.1016/j.fitote.2020.104691 |
| [25] |
ZHOU K, ZHAO X L, HAN L P, et al. Paecilomycines A and B, novel diterpenoids, isolated from insect-pathogenic fungi Paecilomyces sp. ACCC 37762[J]. Helvetica Chimica Acta, 2015, 98(5): 642-649. DOI:10.1002/hlca.201400272 |
 2022, Vol. 46
2022, Vol. 46


