中国科学院海洋研究所主办。
文章信息
- 王聪, 梅显贵, 朱伟明. 2016.
- WANG Cong, MEI Xian-Gui, ZHU Wei-Ming. 2016.
- 海洋链霉菌来源的天然产物
- New Natural Products From the Marine-Derived Streptomyces Actinobacteria
- 海洋科学集刊, 51: 86-124
- Studia Marina Sinica, 51: 86-124.
- http://dx.doi.org/10.12036/hykxjk20160711002
-
文章历史
- 收稿日期:2016-07-11
- 收修改稿日期:2016-07-02
海洋链霉菌由于其独特的生理和代谢功能, 成为海洋微生物活性天然产物的重要来源。本实验室对2010~2013年初海洋放线菌新天然产物的统计表明, 海洋放线菌研究最多的是链霉菌(Streptomyces), 占海洋放线菌新天然产物的60%(赵成英等, 2013)。海洋链霉菌新天然产物的研究始于1976年, Okami课题组报道了首例海洋链霉菌天然产物aplasmomycins A-C (150~152)(Okami et al., 1976); 截至2016年6月的40年时间里, 已报道了547个海洋链霉菌新天然产物。这些天然产物的结构类型包括生物碱、聚酮、萜类、甾体、卤代物、聚醚类等; 并具有多种生物活性, 包括抗肿瘤、抗菌、抗疟和抗寄生虫等。因此, 海洋链霉菌天然产物可能是发现药物先导化合物的重要的资源宝库, 本文将从海洋链霉菌的来源出发, 综述这些天然产物的结构及其生物活性。
1 海洋动物来源的海洋链霉菌天然产物 1.1 海绵来源的海洋链霉菌天然产物海绵来源的(下同)链霉菌Streptomyces sp. Ni-80代谢产生urauchimycins A(1)和B(2), 两个化合物在10 µg/mL时具有抑制白色念珠菌(Canidia albicans)活性(Imamura et al., 1993)。Streptomyces sp. KM86-9B代谢产生具有拓扑异构酶I抑制活性的化合物3~9 (Lee et al., 1998)。环肽dehydroxynocardamine (10)和desmethylenylnocardamine (11)来自Streptomyces sp. M1087, 具有微弱的重组酶sortase B抑制活性, EC50分别为88.3 µg/mL和126.4 µg/mL (Lee et al., 2005)。Streptomyces sp. HB202代谢产生吩嗪类化合物streptophenazines A–H (12~19), 化合物12、14~16和19对枯草芽胞杆菌(Bacillus subtilis)的最小抑菌浓度(minimum inhibitory concentration, MIC)分别为46.9 µg/mL、15.6 µg/mL、62.5 µg/mL、62.5 µg/mL和15.6 µg/mL, 化合物12~14、17和18对金黄色葡萄球菌(Staphylococcus aureus)的MIC分别为62.5 µg/mL、62.5 µg/mL、46.9 µg/mL、62.5 µg/mL和62.5 µg/mL(Mitova et al., 2008)。Streptomyces sp. SpC080624SC-11代谢产生3个吩嗪类化合物20~22(Izumikawa et al., 2010b), Streptomyces sp. Sp080513GE-23代谢产生2个含有氯代吲哚单元的二肽类化合物23和24(Motohashi et al, 2010c), Streptomyces sp. NBRC105896代谢产生化合物25、对人宫颈癌Hela细胞和恶性胸膜间皮瘤细胞ACC-MESO-1的IC50分别为49 µmol/L和88 µmol/L (Izumikawa et al., 2010a), Streptomyces sp. Sp080513GE-26产生2个新的蒽环类化合物26和27、化合物26对Hela细胞和HL-60细胞的IC50分别为120 µmol/L和210 µmol/L (Motohashi et al., 2010a)。Streptomyces sp. SpD081030ME-02代谢产生水杨酰胺衍生物28, 对Hela细胞的IC50为28 µmol/L(Ueda et al., 2010)。联苯蒽糖苷29来自Streptomyces sp. HB202(Schneemann et al., 2010), 化合物29对HepG2、HT-29、GXF251L、LXF529L等8种人肿瘤细胞的IC50为0.13~0.33 µmol/L, 对鼠成纤维细胞NIH-3T3的IC50为0.22 µmol/L, 且对包括耐甲氧西林金黄色葡萄球菌、表皮葡萄球菌(Staphylococcus epidermidis)、人皮肤杆菌和痤疮丙酸杆菌(Propionibacterium acnes)等9种细菌的IC50为2.5~8.4 µmol/L。Tetromycins化合物30~33产自链霉菌S. axinellae Pol001T, 可以抑制布氏锥虫(Trypanosoma bruce) (Pimentel-Elardo et al., 2011)。化合物Lobophorins C (34)和D (35)产自肉质链霉菌S. carnosus AZS17, 化合物34和35对人肝癌细胞株7402的IC50分别为0.6 µg/mL和723.1 µg/mL、对人乳腺癌细胞株MDA-MB-435的IC50分别为61.8 µmol/L和7.5 µmol/L(Wei et al., 2011)。细黄链霉菌(S. microflavus)代谢产生3-乙酰基-5-甲基-2′-脱氧尿嘧啶36(Li et al., 2011); Streptomyces sp. SpD081030SC-03代谢产生小肽JBIR-56 (37)和JBIR-57 (38) (Motohashi et al., 2011)。吲哚生物碱streptomycindole (39)产自链霉菌Streptomyces sp. DA22, 对HL-60、结肠癌HCT-116、卵巢上皮性癌HO-8910和人肝癌细胞HepG2没有活性(Huang et al., 2011)。3个曲古抑菌素40~42是链霉菌Streptomyces sp. RM72的代谢产物, 对组蛋白脱乙酰化酶HDAC1的IC50分别为48 µmol/L、74 µmol/L和57 µmol/L(Hosoya et al., 2012)。环脂肽43~46产自Streptomyces sp. RV15 (Abdelmohsen et al., 2012); 苯并蒽醌糖苷47~49产自Streptomyces sp. BCC45596 (Supong et al., 2012), 其对疟原虫的IC50为0.053 µg/mL、0.142 µg/mL和2.93 µg/mL, 对结核杆菌的MIC分别为3.13 µg/mL、12.50 µg/mL和6.25 µg/mL, 化合物47对KB、MCF-7、NCl-H187和Vero细胞的IC50分别为0.179 µg/mL、0.196 µg/mL、0.092 µg/mL和1.71 µg/mL, 化合物48对KB、MCF-7、NCl-H187和Vero细胞的IC50分别为0.324 µg/mL、0.45 µg/mL、0.242 µg/mL和3.05 µg/mL, 化合物49对KB、MCF-7、NCl-H187和Vero细胞的IC50分别为6.96 µg/mL、3.41 µg/mL、3.97 µg/mL和10.07 µg/mL。链霉菌S. tateyamensis代谢产生化合物JBIR-107(50)(Izumikawa et al., 2013)。吩嗪类化合物streptophenazines I-K (51~53)来自链霉菌Streptomyces HB202, 化合物52对磷酸二酯酶的IC50为12.0 µmol/L, 化合物53对枯草芽胞杆菌、表皮葡萄球菌、磷酸二酯酶的IC50分别为21.6 µmol/L、14.5 µmol/L和12.2 µmol/L (Kunz et al., 2014)。环圈链霉菌S. anulatus S71代谢产生化合物54(Sun et al., 2014b)。Streptomyces sp. M7_15代谢产生monacyclinones A-F (55~60), 化合物57、59和60对横纹肌肉瘤X细胞SJCRH30的IC50分别为160 µmol/L、270 µmol/L和0.73 µmol/L (Vicente et al., 2015)。二肽xestostreptin (61)产自Streptomyces sp. S.4, 其对恶性疟原虫的IC50为50 µmol/L(Rakotondraibe et al, 2015)。化合物quinomycin G (62)和环二肽63来源于Streptomyces sp. LS298, 化合物63对ACHN、786-O、U87 MG和Jurkat肿瘤细胞的IC50分别为0.552 µmol/L、0.721 µmol/L、0.627 µmol/L和0.414 µmol/L(Zhen et al., 2015)。Streptomyces sp. SBT345代谢产生氯代化合物ageloline A (64), 对沙眼衣原体的IC50为9.54 µmol/L (Cheng et al., 2016)。
1.2 珊瑚来源的海洋链霉菌天然产物珊瑚来源的(下同)的链霉菌Streptornyces sp.代谢产生octalactins A (65)和B (66), 化合物65对B-16-FI0和HCT-116细胞的IC50分别为0.0072 µg/mL和0.5 µg/mL (Tapiolas et al., 1991)。多氯代聚酮strepchloritides A (67)和B (68)产自Streptomyces sp. OUCMDZ-1703, 其对MCF-7细胞的IC50分别为9.9 µmol/L和20.2 µmol/L(Fu et al., 2013)。Axinelline A (69)来自链霉菌S. axinellae SCSIO02208, 对环氧化酶COX-2的IC50为2.8mmol/L(Ai et al., 2014)。Nahuoicacids 70~73来自Streptomyces sp. SCSGAA 0027 (Nong et al., 2016)(图 1)。

|
| 图 1 化合物1~73的结构 Fig. 1 Structures of compounds 1~73 |
其他海洋动物来源的(下同)链霉菌S. hygroscopicus代谢产生salinamides A(74)和B(75), 其对肺炎链球菌Streptococcus pneumoniae的MIC均为4 µg/mL、对酿脓葡萄球菌(Staphylococcus pyrogenes)的MIC分别为4 µg/mL和2 µg/mL; 化合物74和75还有抗炎活性, 浓度为59 µg/mL时, 其对佛波醇酯诱导的小鼠耳水肿的抑制率分别为84%和83% (Trischman et al., 1994)。Halichomycin(76)来自链霉菌S. hygroscopicus, 对小鼠白血病P388细胞的ED50为0.13 µg/mL(Takahashi et al., 1994)。大环内酰胺aburatubolactam A (77)产自Streptomyces sp. SCRC-A20, 可抑制TPA诱导的超氧化阴离子的产生(Bae et al., 1996)。吲哚衍生物78~80产自Streptomyces sp. BL-49-58-005 (Sánchez et al., 2003), 化合物78对白血病K562细胞的GI50为8.46 µmol/L; 化合物79对不同肿瘤细胞株表现出中等细胞毒活性, GI50在微摩尔级, 并且无明显选择性; 化合物80对所测试的肿瘤细胞株没有活性。粉蝶霉素piericidins C7(81) and C8(82)来自Streptomyces sp. YM14-060, 可抑制小鼠神经母细胞瘤细胞Neuro-2a增殖而不杀死细胞, IC50分别为0.83 nmol/L和0.2nmol/L (Hayakawa et al., 2007)。Streptomyces sp. JP90代谢产生cinnamoylphosphoramide (83), 其对乙酰胆碱酯酶的IC50为250 µmol/L(Quitschau et al., 2008)。Streptomyces sp. CP32的代谢产生噻唑类衍生物84~88, 化合物84和87可选择性地结合5-HT2B受体(Ki分别为0.50 µmol/L和1.54 µmol/L) (Lin et al., 2010)。化合物89产自Streptomyces sp. BOSC-022A(Lorente et al., 2010)。Nobilamides A-E(90~94)和F-H(95~97)分别产自链霉菌HQ696493和HQ696492, 化合物91具有拮抗TRPV1作用(Lin et al., 2011)。化合物98产自Streptomyces sp., 对P388细胞有细胞毒活性(Mahyudin et al., 2012)。Streptomyces sp. 1053U.I.1a.1b的代谢产生化合物99及其糖苷100(Lin et al., 2012)。化合物violapyrones H (101)和I (102)来自Streptomyces sp. 112CH148, 化合物101对HCT-15细胞的活性最高, GI50为1.10 µg/mL (Shin et al., 2014)。聚酮类lobophorins H (103)和I (104)产自Streptomyces sp. 1053U.I.1a.3b, 化合物104对结核分枝杆菌和枯草杆菌的MIC分别为2.6mmol/L和10.6mmol/L(Lin et al., 2014)。Hyaluromycin (105)来自Streptomyces sp. MB-PO13, 化合物105对透明质酸酶的IC50为14 µmol/L(Harunari et al., 2014)。大环内酯类化合物PM100117 (106)和PM100118 (107)产自链霉菌S. caniferus GUA-06-05-006A, 其对A549细胞的GI50分别为1.3 µmol/L和0.83 µmol/L、对MDA-MB-231细胞的GI50分别为2.7 µmol/L和1.7 µmol/L、对人结肠癌细胞HT29的GI50分别为3.8 µmol/L和9.2 µmol/L (Pérez et al., 2016)。
2 海洋植物来源的海洋链霉菌天然产物 2.1 海藻来源的海洋链霉菌天然产物海藻链霉菌Streptomyces sp. #N1-78-1代谢产生蒽醌类化合物108~110, 3个化合物对金黄色葡萄球菌的IC50分别为0.15 µmol/L、0.36 µmol/L和31 µmol/L(Socha et al., 2006)。化合物streptobactin (111)、dibenarthin (112)和tribenarthin (113)产自海藻链霉菌Streptomyces sp. YM5-799(Matsuo et al., 2011)。
2.2 红树林来源的海洋链霉菌天然产物红树植物来源的链霉菌(下同) Streptomyces sp. GT2002/1503代谢产生吲哚并倍半萜烯xiamycin B (114)、indosespene (115)和sespenine (116), 化合物114具有选择性抑制HIV活性(Ding et al., 2010)(图 2)。
|
| 图 2 化合物74~115的结构 Fig. 2 Structures of compounds 74~115 |
Streptomyces sp. HK10552代谢产生化合物117~120(Wang et al., 2010); 吲哚生物碱121和吲哚生物碱122产自Streptomyces sp. GT2002/1503, 121和122对HT-29、GXF 251L、LXFA 629L等12种人癌细胞的平均IC50值分别为大于30 µmol/L和10.1 µmol/L; 化合物121选择性地抑制HIV病毒的R5受体活性(Ding et al., 2010)。化合物divergolides A-D (123~126)产自Streptomyces sp. HKI0576, 化合物123对肺癌细胞LXFA 629L、胰腺癌细胞PANC-1、肾癌细胞RXF 486L和恶性毒瘤Saos-2的IC50值为1.0~2.0 µmol/L(Ding et al., 2011)。化合物divergolides A-D (127~130)也产自该菌(Xu et al., 2014)。链霉菌S. lusitanus XM52代谢产生131~132, 其中132对金黄色葡萄球菌、枯草杆菌的MIC分别为32 µg/mL和8 µg/mL(Han et al., 2012)。倍半萜类化合物133~137产自放线菌Streptomyces sp. HKI0595, 对12种人肿瘤细胞均无细胞毒活性, 对枯草杆菌和牛分支杆菌有较弱的抑制活性(Ding et al., 2012)。
2.3 其他植物来源的链霉菌天然产物其他海洋植物来源的链霉菌Streptomyces sp. CANU Fox 21-2-6代谢产生化合物138~140, 对小鼠白血病细胞P388的IC50值为0.4~0.06 µg/mL(Phipps et al., 2004)。来自草绿盐角草(Salicornia herbacea)的链霉菌Streptomyces sp. FX-58代谢产生化合物141 (Huang et al., 2006a)和化合物142~143 (Huang et al., 2006b), 化合物143对HL-60、BCTC-823和MDA-MB-435细胞的IC50值分别为6.83 µg/mL、82.2 µg/mL和56.59 µg/mL。黄酮类化合物144产自Streptomyces sp. MA-12, 浓度为0.25mmol/L时, 对香蕉炭疽病菌、小麦赤霉病菌和桔青霉3种植物病原菌有抑制作用(Ding et al., 2013)。不饱和脂肪酸145产自链霉菌S. violans HTTA-F04129, 对A2780细胞的IC50值为4.36 µmol/L(Huang et al., 2014)。Streptomyces sp. LC6代谢产生juanlimycins A (146)和B (147), 可抑制沙门氏菌毒力岛-1的分泌(Zhang et al., 2014a)。来源于浒苔的链霉菌Streptomyces sp. OUCMDZ-3434代谢产生结构新颖的二聚体wailupemycins H (148)和I (149), 其对α-糖苷酶的Ki值分别为16.8 µmol/L和6.0 µmol/L、IC50值分别为19.7 µmol/L和8.3 µmol/L (Chen et al., 2016)。
3 海泥和海水来源的链霉菌天然产物 3.1 海泥来源的链霉菌天然产物海泥来源的链霉菌(下同)S. griseus SS-20代谢产生aplasmomycins A-C (150~152), 能抑制多种革兰氏阳性细菌(包括分枝细菌), 同时具有抗疟原虫的活性。感染了疟原虫的小鼠口服后, 疟原虫大大减少, 且小鼠全部存活, 而未经过治疗的小鼠在8d内全部死亡(Okami et al., 1976; Nakamura et al., 1977)。链霉菌S. tenjimariensis SS-939代谢产生氨基糖苷类化合物istamycins A和istamycins B (153和154), 对革兰氏阳性菌和阴性菌都有很好的抑制作用(Okami et al., 1979)。生物碱altemicidin(155)产自S. sioyaensis SA-1758, 具有抗肿瘤和杀螨活性(Okami et al., 1989)。4个罕见的吩嗪生物碱类化合物156~159来自Streptomyces sp. CNB-253, 化合物156对嗜血杆菌属流感的IC50值可达到1 µg/mL、对梭菌属产气荚膜杆菌IC50值可达到4 µg/mL(Pathirana et al., 1992)。N-乙酰-β-D-氨基葡糖苷酶抑制剂pyrostatins A (160)和B (161)产自Streptomyces sp. SA-3501(Aoyama et al., 1995)。二酮哌嗪maremycins A (162)和B (163)产自Streptomyces sp. B 9173 (Balk-Bindseil et al., 1995)。Streptomyces sp. BD-26T(20)代谢产生wailupemycins A-C (164~166)和3-epideoxyenterocin (167), 化合物167浓度在1mg/6mm disk时, 对金黄色葡萄球菌的抑菌圈为18mm; 化合物164浓度在0.1mg/6mm disk时, 对大肠杆菌E. coli的抑菌圈为15mm (Sitachitta et al., 1996)。多色霉素类抗生素δ-indomycinone 168产自Streptomyces sp. B 8300, 对枯草芽孢杆菌B. subtilis的MIC为100 µg/mL(Biabani et al., 1997)。Actinoflavoside (169)产自Streptomyces sp. CNB-689, 对革兰氏阳性菌肺炎链球菌(S. pneumonia)、酿脓葡萄球菌(S. pyrogenes)、金黄色葡萄球菌和藤黄微球菌(M. luteus)的MIC均为64 µg/mL(Jiang et al., 1997)。Streptomyces sp. B 8251代谢产生生物碱170, 具有微弱的抑制大肠杆菌(E. coli)和枯草芽孢杆菌(B. subtilis)的活性(Pusecker et al., 1997)。Anthranilamide (171)产自Streptomyces sp. B7747, 浓度为100 µg/disk, 对淡水小球藻(C. sorokiniana)的抑制圈直径为11mm、对普通小球藻(C. vulgaris)的抑制圈直径为13mm、对栅藻(S. subspicatus)的抑制圈直径为12mm(Biabani et al., 1998)。Streptomyces sp. BD-18T(41)代谢产生多环苯醌类化合物halawanones A-D(172~175)(Ford et al., 1998)。两个弱的抗菌活性的芳香族胺类化合物lornemides A(176)和B(177)产自Streptomyces sp. MSTMA190, 176对枯草杆菌(B.subtilitis)的LD99为50 µg/mL(Capon et al., 2000)。化合物178~181来自Streptomyces sp. B5632和B3497(Mukku et al., 2000)。化合物182和183分别来自Streptomyces sp. SS99BA-2 (Hernandez et al., 2000)和Streptomyces sp. 1010(Ivanova et al., 2001), 化合物183对藤黄微球菌(M. luteus)和枯草芽孢杆菌(B. subtilis)的MIC分别为15 µg/mL和50 µg/mL, 而对白色念珠菌(C. albicans)和大肠杆菌(E. coli)没有抑制活性。Streptomyces sp. M02750代谢产生化合物184~189, 无抗白色念珠菌(C. albicans)活性(Cho et al., 2001)。大环内酯chalcomycin B (190)产自Streptomyces sp. B7064, 其在浓度为10 µg/9mm disk时, 对金黄色葡萄球菌、大肠杆菌(E. coli)、枯草杆菌(B. subtilis)的抑菌圈直径分别为23mm、28mm和21mm (Asolkar et al., 2002)。化合物parimycin(191)来自Streptomyces sp. B8652, 其对GXF251L、H460、LXFA629L、LXFL529L、MCF-7、MAXF401NL、MEXF462NL和MEXF 514L细胞的IC70为0.9~6.7 µg/mL(Maskey et al., 2002)。化合物gutingimycin(192)也来源于该链霉菌(Maskey et al., 2004)。链霉菌Streptomyces sp. BD21-2代谢产生bonactin (193), 在测试浓度为1mg/mL时, 对金黄色葡萄球菌、芽孢杆菌(B. megaterium)和酿酒酵母菌(S. cerevisiae)的抑菌圈直径分别为7mm、8mm和7.5mm (Schumacher et al., 2003)。Komodoquinone A (194)产自Streptomyces sp. KS3, 在1 µg/mL时, 即可诱导神经母细胞瘤细胞株Neuro 2A的形态变化(Itoh et al., 2003)。内酯类化合物195~196和大环内酰胺类化合物197分别产自Streptomyces sp. B6007 (Stritzke et al., 2004)和S. aureoverticillatus NPS001583(Mitchell et al., 2004), 对HT-29、B16-F10和人外周白血病细胞的EC50分别为3.6 µmol/L、2.2 µmol/L和2.3 µmol/L。
Lajollamycin (198)产自链霉菌S. nodosus NPS007994, 对耐青霉素肺炎双球菌(S. pneumonia)和耐甲氧西林葡萄球菌(S. aureus)的MIC分别为1.5 µg/mL和5 µg/mL, 对小鼠黑色素瘤细胞系B16-F10的EC50为9.6 µmol/L (Manam et al., 2005)。氯代手霉素衍生物chinikomycins A (199)和B (200)来自Streptomyces sp. M045, 化合物199对乳腺癌细胞MAXF 401NL、黑色素瘤细胞MEXF 462NL和肾癌细胞RXF 944L的IC50分别为2.41 µg/mL、4.15 µg/mL和4.02 µg/mL, 化合物200对MAXF 401NL的IC50为3.04 µg/ml(Li et al., 2005)。Streptomyces sp. NPS008187代谢产生glaciapyrroles A-C (201~203), 其中201对结直肠腺癌细胞HT-29和黑色素瘤细胞B16-F10的IC50均为180 µmol/L(Macherla et al., 2005)。拒霉素衍生物204产自Streptomyces sp. B8005, 抑制大肠杆菌(E. coli)、金黄色葡萄球菌和绿色产色链霉菌(S. viridochromogenes)的MIC大于40 µg/mL(Kock et al., 2005)。Actinofuranones A (205)和B(206)产自Streptomyces sp. CNQ766 (Cho et al., 2006a), 该菌株还代谢产生azamerone (207)(Cho et al., 2006b)。Daryamides A-C (208~210)、化合物211来自Streptomyces sp. CNQ-085, 化合物208对HCT-116细胞的IC50为3.15 µg/mL, 化合物208~209对白色念珠菌的MIC分别为62.5 µg/mL和125 µg/mL (Asolkar et al., 2006) (图 3)。

|
| 图 3 化合物116~211的结构 Fig. 3 Structures of compounds 116~211 |
星孢菌素N-carboxamidostaurosporine (212)、倍半萜类213和生物碱类214来自链霉菌Streptomyces sp. QD518, 化合物214对膀胱、中枢神经系统、结肠、胃、头、颈、肺、乳房、胰腺、前列腺, 以及黑色素瘤等37个人癌细胞的平均IC50和IC70值分别为0.016 µg/mL和0.17 µg/mL, 化合物214在40 µg/disc时对金黄色葡萄球菌的抑菌圈直径为11mm(Wu et al., 2006)。Streptomyces sp. CNQ-583代谢产生吡咯生物碱bohemamine B (215)、bohemamine C (216)和5-chlorobohemamine C(217)(Bugni et al., 2006)。化合物urauchimycin C (218)来自Streptomyces sp. B1751(Yao et al., 2006)。Streptokordin (219)产自Streptomyces sp. KORDI-3238, 对细胞MDA-MB-231、HCT15、PC-3、NCI-H23、ACHN、LOX-IMVI、K-562的IC50值分别为7.5 µg/mL、7.8 µg/mL、3.2 µg/mL、3.5 µg/mL、4.7 µg/mL、7.4 µg/mL和8.6 µg/mL(Jeong et al., 2006)。Streptomyces sp. B8000代谢产生化合物220和221, 化合物220在40 µg/disc时, 对金黄色葡萄球菌和绿色链霉菌的抑菌圈直径为分别为14mm和12mm(Poumale et al., 2006)。化合物octalactin C (222)来自Streptomyces sp. V5(陈光英等, 2007)。Streptomyces sp. M491代谢产生倍半萜化合物223~225(Wu et al., 2007)和226~231(Ding et al., 2009), 化合物226对多种肿瘤细胞的平均IC50为6.7 µg/mL。Streptomyces sp. CNH990代谢产生醌类化合物marmycins A (232)和marmycins B(233), 化合物232对人结肠癌细胞HCT-116的IC50为60.5nmol/L, 是其氯代同系物227的18倍(IC50为1.09 µmol/L), 232还具有G1期阻滞活性(Martin et al., 2007)。环六肽piperazimycins A-C (234~236)产自链霉菌Streptomyces sp. CNQ-593, 其对人结肠癌细胞HCT-116的平均GI50均为76ng/mL (Miller et al., 2007)。吡咯生物碱marinopyrroles A (237)和B (238)来自链霉菌Streptomyces sp. CNQ-418, 其对耐甲氧西林金黄色葡萄球菌(MRSA)的MIC90分别为0.61 µmol/L和1.1 µmol/L、对HCT-11细胞的IC50分别为8.8 µmol/L和9.0 µmol/L(Hughes et al., 2008); 对该菌株优化后又得到marinopyrroles C-F (239~245), 对抑制人结肠癌HCT-116细胞的IC50为1~5 µg/mL, 化合物239抑制MRSA的MIC小于1 µg/mL(Hughes et al., 2010)。Streptomyces sp. MS239代谢产生吲哚类化合物243和萜类化合物244, 化合物243对枯草杆菌ATCC6633有弱的抑菌活性(Motohashi et al., 2008)。化合物essramycin (245)来自于Streptomyces sp. Merv8102, 其对革兰阳性菌和革兰阴性菌的MIC为1.0~8.0 µg/ml。对大肠杆菌(ATCC 10536)、铜绿假单胞菌(ATCC 10145)、枯草杆菌(ATCC 6051)、金黄色葡萄球菌(ATCC 6538)和藤黄微球菌(ATCC 9341)的MIC分别为8 µg/mL、3.5 µg/mL、1 µg/mL、1 µg/mL和1.5 µg/mL (El-Gendy et al., 2008)。
Streptomyces sp H668代谢产生聚醚类化合物246, 有抗疟疾活性, IC50为100~200ng/mL (Na et al., 2008)。有机酸247和248来自Streptomyces sp. Act8015 (Shaaban et al., 2008)。聚酮phaechromycins F-H (249~251)产自Streptomyces sp. DSS-18(Li et al., 2008)。Streptomyces sp. CNQ-617代谢产生marineosins A和B(252~253), 其对人结肠癌细胞HCT-116的IC50分别为0.5 µmol/L和46 µmol/L (Boonlarppradab et al., 2008); 对marineosins的生物合成研究, 又从该链霉菌的代谢产物中获得了化合物254~257, 其中256对HepG2细胞的IC50为4.17 µmol/L; 化合物256对氯喹敏感(CQS)、氯喹拮抗, 以及采用氯喹处理过的菌株P. falciparum的IC50分别为2.3nmol/L、12nmol/L和1.5nmol/L (Salem et al., 2014)。三环聚丙酸酯类indoxamycins A-F (258~263)产自Streptomyces sp. NPS-643, 化合物258和263对人结肠癌细胞HT-29的IC50为0.3~3 µmol/L(Sato et al., 2009)。氯代生物碱ammosamides A (264)和B (265)产自链霉菌Streptomyces sp. CNR-698, 264暴露在空气中可逐渐转化为265, 其对人结肠癌HCT-116细胞的IC50均为320nmol/L, 作用靶点为肌球蛋白(Hughes et al., 2009)。化合物splenocins A-J (266~275)产自Streptomyces sp. CNQ431, 抑制脾脏细胞因子的IC50为2~50nmol/L, 且对哺乳动物细胞的细胞毒性较小, 具有治疗哮喘的潜力(Strangman et al., 2009)。Streptomyces sp. NTK 227代谢产生albidopyrone A (276), 具有蛋白酪氨酸磷酸酶B(PTP1B)抑制活性, IC50为128 µg/ml(Hohmann et al., 2009)。细胞毒活性的mansouramycins A-D (277~280)和tartrolon D (281)分别产自Streptomyces sp. Mei37(Hawas et al., 2009)和Streptomyces sp. MDG-04-17-069(Pérez et al., 2009), 化合物281对A549、HT29和MDA-MB-231的GI50分别为0.16 µmol/L、0.31 µmol/L和0.79 µmol/L。二酮哌嗪naseseazines A (282)和B (283)产自Streptomyces sp. CMB-MQ030 (Raju et al., 2009)。Lorneic acids A (284)和B (285)产自Streptomyces sp. NPS554, 其抑制人血小板磷酸二酯酶5(PDE5)的IC50分别为12.6 µmol/L和87.1 µmol/L(Iwata et al., 2009); 多环聚酮类化合物akaeolide (286)也产自该菌, 其对大鼠成纤维细胞3Y1的IC50为8.5 µmol/L(Igarashi et al., 2013)。Tirandamycins C (287)和D (288)产自Streptomyces sp. 307-9, 抑制耐万古霉素肠球菌(VRE)的MIC分别为110 µmol/L和大于9 µmol/L(Carlson et al., 2009)。化合物289来自S. xiamenensis 318, 具有治疗肺纤维化的潜力(Xu et al., 2012)。肿大链霉菌(S. tumescens) YM23-260产生环肽类化合物290和291, 化合物290在浓度为100 µmol/L时, 具有抑制阿尔兹海默病活性(Motohashi et al., 2010b)。环肽类化合物292产自Streptomyces sp. MWW064, 具有抑制鼠肿瘤细胞26-L5的转移作用(Igarashi et al., 2010)。Streptomyces sp. CMB-M0406产生heronamides A-C (293~295) (Raju et al., 2010a)和8-deoxyheronamide C (296)(Sugiyama et al., 2014), 296对裂殖酵母细胞的MIC可达5.8 µmol/L。化合物297~299为Streptomyces sp. CMB-M0423的代谢产物, 对革兰阳性菌的IC50为0.6~6.5 µmol/L(Raju et al., 2010b)(图 4)。
|
| 图 4 化合物212~298的结构 Fig. 4 Structures of compounds 212~298 |
化合物300~304产自Streptomycetaceae CNQ-509, 化合物300、302和303对HCT-116细胞的IC50分别为31.1 µmol/L、31.0 µmol/L和5.7 µmol/L(Kwon et al., 2010); Streptomyces sp. B6219代谢产生化合物305(Abdalla et al., 2010)。吡喃酮类化合物306~309产自S. albus POR-04-15-053, 化合物306和309对MDA-MB-231、HT-29和A549的GI50为0.24~0.69 µmol/L(Schleissner et al., 2011)。抗霉素antimycins A19和A20 (310~311)产自S. antibioticus H74-18, 其抑制白色念珠菌(C. albicans)的MIC为5~10 µg/mL(Xu et al., 2011)。Streptomyces. sp CNQ-027代谢产生actinoramides A-C (312~314)(Nam et al., 2011)和actinoranone (315)(Nam et al., 2013), 化合物315对HCT-116细胞的LD50为2.0 µg/mL。Streptomyces sp. RJA2928代谢产生pandanamide B (316) (Williams et al., 2011)、nahuoic acid A (317) (Williams et al., 2013)和nahuoic acids D-E (318~319)(Williams et al., 2016), 化合物316抑制T淋巴细胞细胞的IC50为20 µg/mL、化合物317和319对组蛋白甲基化酶的IC50分别6.5 µmol/L和13 µmol/L。缩酚酸肽fijimycins A-C (320~322)来自Streptomyces sp. CNS-575, 其对MRSA (ATCC33591)、Sanger 252和UAMS1182的MIC100为4~16 µg/mL(Sun et al., 2011)。Streptomyces sp. 211726代谢产生化合物323~324(Yuan et al., 2011)和325~331(Yuan et al., 2013), 对白色念珠菌(C. albicans)和人结肠癌细胞HCT-116有抑制活性。化合物332产自Streptomyces sp. 061316 (Chen et al, 2011)。安沙霉素类化合物ansalactam A (333)来自Streptomyces sp. CNH-189(Wilson et al., 2011); 该菌还代谢产生混源萜334~337 (Kaysser et al., 2012)和ansalactams B-D (338~340)(Le et al., 2016), 化合物338~340对MRSA的MIC分别为31.2 µg/mL、31.2 µg/mL和62.5 µg/mL。化合物benzoxacystol (341)来自Streptomyces sp. NTK 935, 抑制糖原合成酶激酶GSK-3β的IC50为1.35 µmol/L, 对小鼠成纤维细胞NIH-3T3有弱抑制活性(Nachtigall et al., 2011)。Streptomyces sp. B8112代谢产生glucopiericidin C (342), 有抗米黑毛霉菌活性和细胞毒活性(Shaaban et al., 2011)。Streptomyces sp. MST-MA568代谢产生螺环缩酮reveromycin E (343)(Fremlin et al., 2011)。吡喃酮A-C (344~346)产自Streptomyces sp. CNQ-301 (Fukuda et al, 2011)。Streptomyces sp. WuXin代谢产生化合物347和348, 对HL-60有细胞毒活性(Li et al., 2011); 化合物usabamycin A-C (349~351)产自Streptomyces sp. NPS853, 对人宫颈癌HeLa细胞有弱细胞毒活性并可选择性抑制对5-羟色胺的摄取(Sato et al., 2011)。Streptomyces sp. W007代谢产生化合物352, 对A549细胞有细胞毒活性(Zhang et al., 2011)(图 5)。

|
| 图 5 化合物299~352的结构 Fig. 5 Structures of compounds 299~352 |
化合物353产自杆菌状链霉菌S. bacillaris SNB-019(Hu and MacMillan, 2012)。对弗氏链霉菌S. fradiae 007进行紫外照射和亚硝基胍诱变, 得到突变株S. fradiae 007M135, 该突变株代谢产生吲哚咔唑生物碱354~356, 其对HL-60、K562、A549、BEL-7402细胞均有抑制活性, 对蛋白激酶C (PKC-α)的IC50值为0.001~4.6 µmol/L(Fu et al., 2012a)。糖苷类化合物357~361来自S. lusitanus SCSIO LR32, 357~360对多个肿瘤细胞的IC50值为1.1~31 µmol/L (Huang et al., 2012)。Streptomyces sp. CMB-M0392代谢产生化合物362, 对枯草杆菌ATCC6052和ATCC6633的IC50分别为8 µmol/L和14 µmol/L (Raju et al., 2012)。化合物363和364产自Streptomyces sp. CNQ343, 化合物363对白色念珠菌有较强的抑制活性(Kim et al., 2012)。Streptomyces sp. SCSIO 03032代谢产生吲哚生物碱365~368(Zhang et al., 2012)、indimicins A-E (369~373)、lynamicin F (374)和lynamicin G (375)(Chen et al., 2014), 以及piericidin A1 (376)(Zhang et al., 2014a)、heronamides D-F (377~379)(Zhang et al., 2014b), 化合物366~368对多种肿瘤细胞的IC50为4~15 µmol/L、370对MCF-7的IC50为10.0 µmol/L。吩嗪类化合物380为Streptomyces sp. LB173的代谢产物, 有微弱的抗菌活性, 是一种乙酰胆碱酯酶抑制剂(Ohlendorf et al., 2012)。化合物381产自Streptomyces sp. M268(Xie et al., 2012); 化合物382~385产自Streptomyces sp. SCSIO 02999(Zhang et al., 2012), 4个化合物对致病菌的MIC分别为E.coli ATCC 25922(8 µg/mL、8 µg/mL、16 µg/mL和4 µg/mL)、S.aureus ATCC29 213(8 µg/mL、16 µg/mL、16 µg/mL、8 µg/mL)、B.subtilis SCSIO BS01(64 µg/mL、128 µg/mL、大于128 µg/mL、64 µg/mL)和B. thuringiensis SCSIO BT01(64 µg/mL、64 µg/mL、大于128 µg/mL、大于128 µg/mL)。化合物386产自Streptomyces sp. CP13-10, 对酵母Sir2p基因的MIC为2.5mmol/L、对人类SIRT1和SIRT2基因的IC50分别为3.7 mmol/L和4.5mmol/L(Amagata et al., 2012)。
S. variabilis SNA-020代谢产生生物碱387, 387对胰腺细胞MIA PaCa-2的IC50为3.2 µmol/L(Pan et al., 2012)。化合物388产自Streptomyces sp. TP-A0879, 对小鼠结肠癌细胞26-L5的IC50为0.25 µmol/L(Igarashi et al., 2012)。吲哚咔唑生物碱389和390产自Streptomyces sp. FMA, 化合物390对HL-60、A549和Hela细胞的IC50值分别为1.4 µmol/L、5.0 µmol/L和34.5 µmol/L(Fu et al., 2012b)。Streptomyces sp. CNT-179代谢产生cyanosporasides D-F (391~393)(Lane et al., 2013); S. antibioticus PTZ0016代谢产生394~396, 化合物394~396对金黄色葡萄球菌ATCC6538的MIC分别为6.0mg/mL、6.0mg/mL和8.0mg/mL(Lian and Zhang, 2013)。生物碱nitrosporeusines A (397)和B (398)产自S. nitrosporeus CQT1424, 具有抑制甲型H1N1流感病毒活性(Yang et al., 2013a)。S. niveus SCSIO 3406代谢产生倍半萜marfuraquinocins A-D (399~402)、phenaziterpenes A (403)和B (404), 化合物399和401对肺癌细胞NCI-H460的IC50值分别为3.7 µmol/L和4.4 µmol/L, 化合物399、401和402对金黄色葡萄球菌ATCC292138的MIC值均为8 µg/mL; 化合物401和402对耐甲氧西表皮葡萄球菌shhs-E1的MIC值为8 µg/mL(Song et al., 2013)。Streptomyces sp. CNH-287代谢产生chlorizidine A (405), 其对HCT-116细胞的IC50为3.2~4.9 µmol/L (Alvarez-Mico et al., 2013)。脱氢二酮哌嗪406~410产自Streptomyces sp. FXJ7.328, 化合物407抑制H1N1病毒的IC50为41.5 µmol/L(Wang et al., 2013)。Streptomyces sp. MS100061代谢产生lobophorin G1 (411), 其对枯草芽胞杆菌和结核分支杆菌H37Rv的MIC分别为3.1 µg/mL和32 µg/mL(Chen et al., 2013)(图 6)。

|
| 图 6 化合物353~410的结构 Fig. 6 Structures of compounds 353~410 |
Streptomyces sp. SNJ013代谢产生化合物sungsanpin (412)(Um et al., 2013a)。Streptomyces sp. CNQ-329代谢产生napyradiomycins A-E(413~417), 化合物413、416和417对HCT-116细胞的IC50为4.2 µg/mL、16.1 µg/mL和4.8 µg/mL, 化合物413和414对MRSA的MIC分别为26 µg/mL和64 µg/mL(Cheng et al., 2013)。Streptomyces sp. CNH-070产生napyradiomycin F (418), 其对HCT-116细胞的IC50为9.42 µg/mL(Cheng et al., 2013)。Streptomyces sp. SCSIO 10428代谢产生化合物419~421, 化合物419对金黄色葡萄球菌ATCC29213、枯草芽胞杆菌SCSIO BS01和苏云金杆菌SCSIO BT01的MIC分别为4 µg/mL、4 µg/mL和8 µg/mL, 化合物420对金黄色葡萄球菌ATCC29213、枯草芽胞杆菌SCSIO BS01和苏云金杆菌SCSIO BT01的MIC分别为0.5 µg/mL、1 µg/mL和1 µg/mL, 化合物421对枯草芽胞杆菌SCSIO BS01和苏云金杆菌SCSIO BT01的MIC分别为8 µg/mL和16 µg/mL (Wu et al., 2013a)。化合物anthracimycin (422)来自Streptomyces sp. CNH365, 对炭疽芽胞杆菌UM23C1-1、金黄色葡萄球菌ATCC 13709、粪肠球菌ATCC 29212、肺炎链球菌ATCC 51916、流感嗜血杆菌ATCC 31517KO的MIC分别为0.03125 µg/mL、0.0625 µg/mL、0.125 µg/mL、0.25 µg/mL和4 µg/mL(Jang et al., 2013)。环肽surugamides A-E (423~427)来自Streptomyces sp. JAMM992, 对牛组织蛋白酶B的IC50分别为21 µmol/L、27 µmol/L、36 µmol/L、18 µmol/L和16 µmol/L(Takada et al., 2013)。Streptomyces sp. SCSIO 03219代谢产生萜类化合物03219A (428) (Zhang et al., 2013)。Streptomyces sp. Eg25代谢产生maroxazinone (429), 对MCF7、HEPG2和HCT116细胞的IC50分别为4.32 µg/mL、2.90 µg/mL和8.51 µg/mL(Abdelfattah, 2013)。Streptomyces sp. CNT-372代谢产生farnesides A (430)和B (431), 430对恶性疟原虫的IC50为69.3 µmol/L(Zafrir et al., 2013)。Streptomyces sp. 7-145代谢产生化合物432和433, 对耐甲氧西林金黄色葡萄球菌和耐万古霉素肠球菌有很好的抗菌活性(Wu et al., 2013b)。环肽ohmyungsamycin A(434)和ohmyungsamycin B(435)产自链霉菌Streptomyces sp. SNJ042, 化合物434对枯草芽胞杆菌ATCC6633、藤黄微球菌NBRC12708和变形杆菌NBRC3851的MIC分别为4.28 µmol/L、1.07 µmol/L和2.14 µmol/L, 化合物435对藤黄微球菌NBRC12708的MIC为8.5 µmol/L, 化合物434对HCT116、A549、SNU-638、MDAMB-231和SKHEP-1细胞的IC50分别为0.359 µmol/L、0.551 µmol/L、0.532 µmol/L、0.688 µmol/L和0.816 µmol/L(Um et al., 2013b)。Separacenes A-D(436~439)产自Streptomyces sp. SNJ210, 化合物436对白色念珠菌异柠檬酸裂解酶、人癌细胞HCT-116和A549有弱活性(Bae et al., 2013)。Streptomyces sp. 12A35代谢产生lobophorins H (440)和I (441), 化合物440对金黄色葡萄球菌ATCC29213和枯草芽胞杆菌CMCC63501的MIC分别为50 µg/mL和1.57 µg/mL(Pan et al., 2013)。Strepsesquitriol (442)来自Streptomyces sp. SCSIO 10355, 具有抑制TNF-α的活性(Yang et al., 2013b)。Mollemycin A (443)产自Streptomyces sp. CMBM0244, 对金黄色葡萄球菌ATCC 25293和ATCC 9144、表皮葡萄球菌ATCC12228、枯草芽胞杆菌ATCC 6051和ATCC 6633、大肠杆菌ATCC 25922、绿脓杆菌ATCC 27853和牛结核杆菌的IC50值分别为50nmol/L、10nmol/L、50nmol/L、10nmol/L、10nmol/L、10nmol/L、50nmol/L和3200nmol/L; 对药敏型和多药耐药的恶性疟原虫的IC50分别为9nmol/L和7nmol/L, 活性远大于氯喹, 与青蒿素相当(Raju et al., 2014)。S. drozdowiczii SCSIO 10141代谢产生marformycins A-F (444~449), 化合物444~448对藤黄微球菌的MIC分别为0.25mg/mL、4.0mg/mL、0.25mg/mL、0.063mg/mL和4.0mg/mL (Zhou et al., 2014)。S. nitrosporeus YBH10-5代谢产生nitrosporeunols A-G (450~456)(Liu et al., 2014)。S. scopuliridis SCSIO ZJ46代谢产生化合物457~459, 457对金黄色葡萄球菌ATCC 29213、肺炎双球菌NCTC 7466、耐甲氧西林表皮葡萄球菌的MIC值分别为16.0 µg/mL、12.5 µg/mL和32.0 µg/mL (Song et al., 2014)(图 7)。
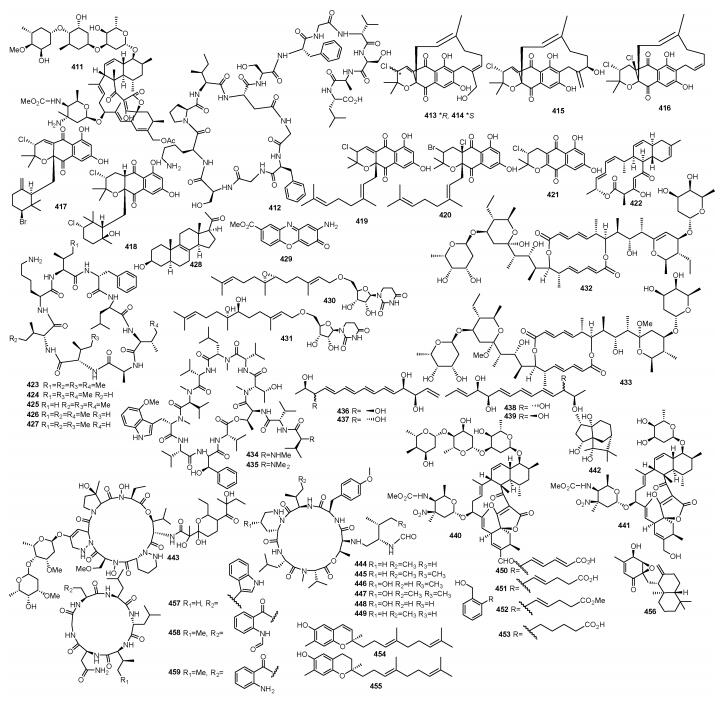
|
| 图 7 化合物411~459的结构 Fig. 7 Structures of compounds 411~459 |
Streptomyces sp. LZ35DgdmAI代谢产生echosides A-E (460~464), 化合物460有很明显的拓扑异构酶Ⅰ活性, 化合物462有拓扑异构酶Ⅱa活性(Deng et al., 2014)。化合物465~468产自Streptomycetaceae CNQ525, 可以诱导HCT-116细胞凋亡(Farnaes et al., 2014)。Streptomyces sp. ACT232代谢产生ahpatinin Ac (469)和ahpatinin Pr (470), 对胃蛋白酶的IC50为11~50nmol/L (Sun et al., 2014a)。Carpatamides A-C(471~473)来自Streptomyces sp. SNE-011, 化合物471和473对NSCLC、HCC366、A549和HCC44细胞的IC50为2.2~8.4 µmol/L (Fu et al., 2014)。化合物anmindenols A (474)和B (475)产自Streptomyces sp. CMDD10D111, 抑制NO产生的IC50值为23 µmol/L和19 µmol/L (Lee et al., 2014)。Arcticoside (476)和C-1027 chromophore-V (477)产自Streptomyces sp. ART5, 化合物476和477对白色念珠菌异柠檬酸(裂合)酶的IC50分别为30.4 µmol/L和37.9 µmol/L; 化合物477对癌细胞MDA-MB231和HCT-116的IC50分别为0.9 µmol/L和2.7 µmol/L(Moon et al., 2014)。Lajollamycins B−D (478~480)产自链霉菌Streptomyces sp. SMC72, 对白色念珠菌异柠檬酸裂解酶的IC50分别为40 µmol/L、50 µmol/L和120 µmol/L (Ko et al., 2014)。Streptomyces sp. SNM55代谢产生mohangamides A (481)和B (482)(Bae et al., 2015b)、hormaomycins B (483)和C (484) (Bae et al., 2015a), 化合物481和482对白色念珠菌ICL的IC50分别为4.4 µmol/L和20.5 µmol/L, 化合物483/484对金黄色葡萄球菌ATCC 25923、枯草芽胞杆菌ATCC 6633、藤黄微球菌NBRC 12708、化脓性链球菌ATCC 19615、肠道沙门菌ATCC 14028和P. hauseri NBRC 3851的MIC分别为7/7 µmol/L、14/56 µmol/L、0.4/0.23 µmol/L、14/8 µmol/L、29/114 µmol/L和29/14 µmol/L。Streptomyces sp. IFM 11440代谢产生nubosins A-C (485~487), 化合物486具有Ngn2基因启动子的活性、促进神经干细胞分化基因有关的mRNA的表达(Arai et al., 2015)。Streptomyces sp. SCSIO 11594代谢产生marangucyclines A (488)和B (489), 化合物489对A549、CNE2、MCF-7、HepG2和HL7702细胞的IC50分别为0.45 µmol/L、0.56 µmol/L、0.24 µmol/L、0.43 µmol/L和3.67 µmol/L (Song et al., 2015)。Suncheonosides A-D (490~493)来自Streptomyces sp. SSC21, 化合物490、491和493能促进脂联素的产生, 具有降糖的潜力(Shin et al., 2015)。二酮哌嗪494产自Streptomyces sp. SCSIO 04496(Luo et al., 2016)。Rocheicoside A (495)和rocheicoside 496产自S. rochei 06CM016, 化合物495对大肠杆菌O157: H7 RSKK 234、耐甲氧西林金黄色葡萄球菌DSM 11729和人白色念珠菌DSM 5817的MIC分别为16 µg/mL、8 µg/mL和4 µg/mL; 化合物496对这3株致病菌的MIC分别为16 µg/mL、16 µg/mL和8 µg/mL (Aksoy et al., 2016)。Streptomyces sp. IFM11490代谢产生elmonin (497)、elmenols A (498)和elmenols B (499), 化合物497和499对AGS细胞有弱细胞毒活性(Yixizhuoma et al., 2015)。Streptomyces sp. SNB-048代谢产生spinoxazines A (500)和spinoxazines B (501)、bohemamine D-I (502~507)(Fu et al., 2016)。Streptomyces sp. CHQ-64代谢产生drimentine I (508), 对Hela的IC50为16.7 µmol/L(Che et al., 2016)。Streptomyces sp. 10A085代谢产生anithiactins A-C (509~511), 其对乙酰胆碱酯酶的IC50分别为63 µmol/L、53 µmol/L和68 µmol/L(Kim et al., 2014)(图 8)。
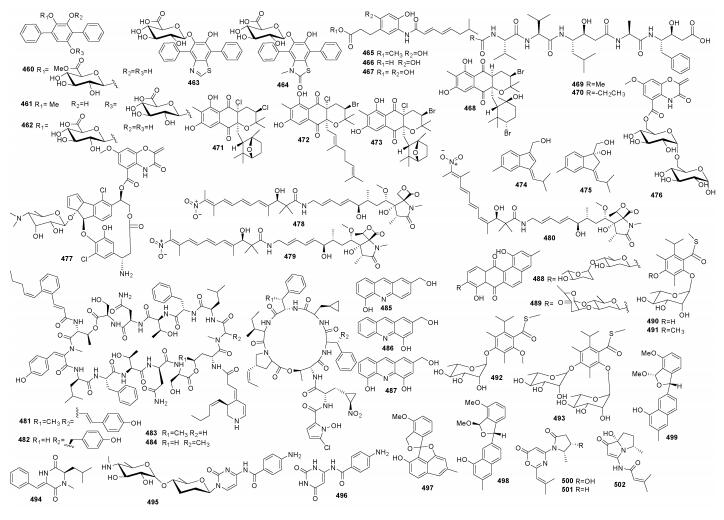
|
| 图 8 化合物460~502的结构 Fig. 8 Structures of compounds 460~502 |
多环蒽醌512和513来自Streptomyces sp. 182SMLY, 对C6、U251、U87-MG和SHG-44c 4种胶质瘤细胞的IC50为0.5~7.3 µmol/L; 化合物512对S. aureus的MIC为20.0 µmol/L(Liang et al., 2016b); 该菌株还代谢产生吩嗪类化合物514~516(Liang et al., 2016a)。Mohangic acids A-E (517~521)来自Streptomyces sp. SNM31, 化合物521对醌还原酶具有良好的活性, 当浓度为20 µmol/L时, 活性是阳性药β-萘黄酮的2.1倍(Bae et al., 2016)。Actinoquinolines A (522)和B (523)产自Streptomyces sp. CNP975, 对环氧化酶COX-1的IC50分别为7.6 µmol/L和2.13 µmol/L, 对环氧化酶COX-2的IC50分别为24.9 µmol/L和1.42 µmol/L (Hassan et al., 2016)。麦角脂醇类化合物ananstreps A-C (524~526)来自S. anandii H41-59, 其中化合物526对SF-268、MCF-7和NCI-H460的IC50分别为13.0 µg/mL、18.1 µg/mL和23.5 µg/mL (Zhang et al., 2016)。
3.2 海水来源的链霉菌天然产物海水来源的链霉菌(下同)Streptomyces sp. Z00045代谢产生cycloheximide acid A (527) (Xu et al., 2013)。Streptomyces sp. F001代谢产生diazaquinomycins E-G (528~530), 528对卵巢癌OVCAR5细胞的IC50为9.0 µmol/L (Mullowney et al., 2014)。Streptomyces sp. TPU1236A代谢产生streptcytosines A-E (531~535), 531对耻垢分枝杆菌的MIC为32 µg/mL (Bu et al., 2014)。一株gntR基因突变的链霉菌代谢产生化合物536~538 (Liu et al., 2015)。
4 未知来源的海洋链霉菌天然产物来源未知的链霉菌(下同)Streptomyces sp. GWS-BW-H5产生内酯类化合物539~544 (Dickschat et al., 2005)。降倍半萜545来自Streptomyces sp. 0616208, 对肝瘤细胞SMMC-7721有细胞毒活性(Xie et al., 2006)。吩嗪类化合物546和547产自Streptomyces sp. CNS284, 其对TNF-α介导的NFκB有抑制作用, IC50分别为4.1 µmol/L和24.2 µmol/L; 抑制LPS介导的NO的IC50分别为48.6 µmol/L和15.1 µmol/L; 对细胞生长和调节因子——前列腺素E2(PGE2)有抑制作用, IC50分别是7.5 µmol/L和0.89 µmol/L(Kondratyuk et al., 2012)(图 9)。
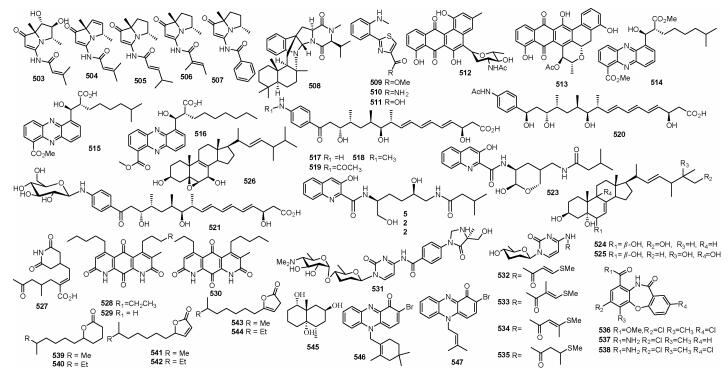
|
| 图 9 化合物503~547的结构 Fig. 9 Structures of compounds 503~547 |
综上所述, 从1976年报道的首例海洋链霉菌来源的aplasmomycins A-C(Okami et al., 1976)到2016年6月的40年时间里, 一共报道了547个海洋链霉菌新天然产物(表 1)。其结构类型呈现多样性, 涉及含氮化合物, 如生物碱、聚酮、萜类、甾体、糖苷、聚醚类及其卤代物等, 且67.3%的化合物表现出肿瘤细胞毒、抗菌、抗疟、抗寄生虫, 以及糖苷酶抑制等生物活性(表 1), 是发现海洋活性化合物的重要资源。
|
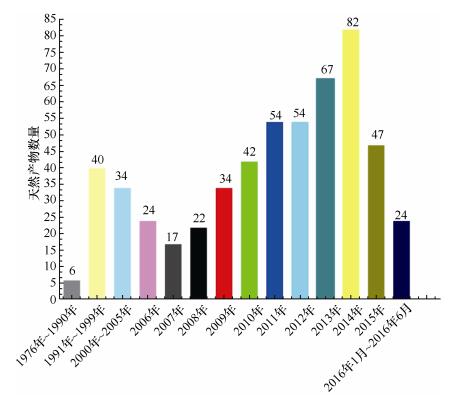
(1)从海洋链霉菌NPs的数量看, 新化合物的数量呈现年递增的趋势。从2006年开始, 新化合物递增的趋势越来越显著, 2014年最多, 报道了82个(图 10)。
(2)从海洋链霉菌的样品来源看, 产生新化合物最多的海洋链霉菌的来源依次是海泥(377个)、海绵(64个)和其他海洋动物(34个), 分别占68.9%、11.7%和6.2%(图 11)。
(3)从化合物的结构类型看, 化合物最多的类型依次是含氮(336个)和聚酮(196个), 分别占海洋链霉菌NPs总数的61.4%和35.8% (图 12)。
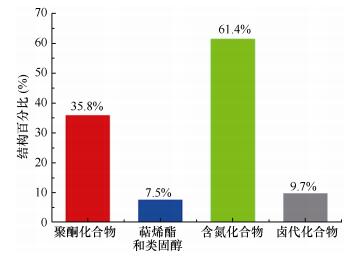
|
| 图 12 海洋链霉菌天然产物的结构分类 Fig. 12 The main structure types of marine-derived Streptomyces actinomycetes MNPs |
(4)约67.3%的海洋链霉菌NPs(368个)表现出肿瘤细胞毒、抗菌、抗疟和抗寄生虫等生物活性, 而肿瘤细胞毒活性(141个)和抑菌活性(117个)是主要的活性类型, 分别占活性化合物总数的38.3%和31.8%(图 13)。
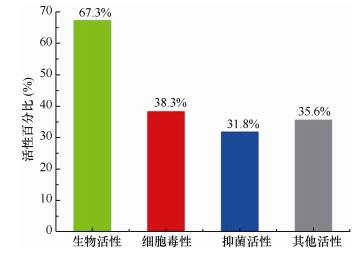
|
| 图 13 海洋链霉菌天然产物的活性分类 Fig. 13 Bioactive categories of marine-derived Streptomyces actinomycetes MNPs |
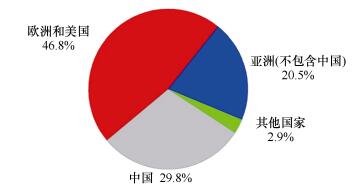
(5)欧美、中国和其他亚洲国家是海洋链霉菌NPs的主要发现者, 其发表化合物的数量分别为256个、163个和112个, 我国学者贡献了29.8%的海洋链霉菌NPs(图 14); 欧美、中国和其他亚洲国家的学者分别发表了93篇、63篇和52篇文章, 其他国家发表了9篇相关文章, 分别占42.9%、29.0%、24.0%和4.1%。
(6)我国学者在63篇学术文章中贡献了163个海洋链霉菌NPs, 在天然产物化学类、有机化学类和海洋药物类的主流杂志, 如J Nat Prod、Org Lett、J Org Chem和Mar Drugs的学术文章分别有11篇、7篇、1篇和13篇, 占其文章总数的17.5%、11.1%、1.6%和20.6% (图 15)。
| 陈光英, 朱峰, 林永成. 2007. 海洋放线菌Streptomyces sp. V5产生的一个新的八元环内酯.有机化学, 27 (9) : 1159–1161 |
| 赵成英, 朱统汉, 朱伟明. 2013. 2010-2013之海洋微生物新天然产物. 有机化学, 33 (6) : 1195–1234 |
| Abdalla M A, Helmke E, Laatsch H. 2010. Fujianmycin C, A bioactive angucyclinone from a marine derived Streptomyces sp. B6219. Nat Prod Commun, 5 (12) : 1917–1920 |
| Abdelfattah M S. 2013. A new bioactive aminoph-enoxazinone alkaloid from a marine-derived actinomycete. Nat Prod Res, 27 (22) : 2126–2131 DOI:10.1080/14786419.2013.793686 |
| Abdelmohsen U R, Zhang G L, Philippe A, et al. 2012. Cyclodysidins A-D, cyclic lipopeptides from the marine sponge-derived Streptomyces strain RV15. Tetrahedron Lett, 53 (1) : 23–29 DOI:10.1016/j.tetlet.2011.10.051 |
| Ai W, Lin X P, Tu Z C, et al. 2014. Axinelline A, a new COX-2 inhibitor from Streptomyces axinellae SCSIO02208. Nat Prod Res, 28 (16) : 1219–1224 DOI:10.1080/14786419.2014.891204 |
| Aksoy S?, Uzel A, Bedir E. 2016. Cytosine-type nucleosides from marine-derived Streptomyces rochei 06CM016. J Antibiot (Tokyo), 69 (1) : 51–56 DOI:10.1038/ja.2015.72 |
| Alvarez-Mico X, Jensen P R, Fenical W, et al. 2013. Chlorizidine, a cytotoxic 5H-pyrrolo[2, 1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp.. Org Lett, 15 (5) : 988–991 DOI:10.1021/ol303374e |
| Amagata T, Xiao J, Chen Y P, et al. 2012. Creation of an HDAC-based yeast screening method for evaluation of marine-derived actinomycetes: discovery of streptosetin A. J Nat Prod, 75 (12) : 2193–2199 DOI:10.1021/np300640g |
| Aoyama T, Kojimal F, Imada C, et al. 1995. Pyrostatins A and B, new inhibitors of N-acetyl-β-D-glucosaminidase, produced by Streptomyces sp. SA-3501. J Enzyme Inhib, 8 (4) : 223–232 |
| Arai M A, Koryudzu K, Ishibashi M. 2015. Inubosins A, B, and C are acridine alkaloids isolated from a culture of Streptomyces sp. IFM 11440 with Ngn2 promoter activity. J Nat Prod, 78 (2) : 311–314 |
| Asolkar R N, Jensen P R, Kauffman C A, et al. 2006. Daryamides A-C, weakly cytotoxic polyketides from a marine-derived actinomycete of the genus Streptomyces Strain CNQ-085. J Nat Prod, 69 (12) : 1756–1759 DOI:10.1021/np0603828 |
| Asolkar R N, Maskey R P, Helmke E, et al. 2002. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J Antibiot (Tokyo), 55 (10) : 893–898 DOI:10.7164/antibiotics.55.893 |
| Bae M, Chung B, Oh K B, et al. 2015a. Hormaomycins B and C: new antibiotic cyclic depsipeptides from a marine mudflat-derived Streptomyces sp. . Mar Drugs, 13 (8) : 5187–5200 DOI:10.3390/md13085187 |
| Bae M, Kim H, Moon K, et al. 2015b. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org Lett, 17 (3) : 712–715 DOI:10.1021/ol5037248 |
| Bae M, Kim H, Shin Y, et al. 2013. Separacenes A-D, novel polyene polyols from the marine actinomycete, Streptomyces sp. . Mar Drugs, 11 (8) : 2882–2893 DOI:10.3390/md11082882 |
| Bae M, Moon K, Kim J, et al. 2016. Mohangic Acids A-E, p-aminoacetophenonic acids from a marine mudflat-derived Streptomyces sp. . J Nat Prod, 79 (2) : 332–339 DOI:10.1021/acs.jnatprod.5b00956 |
| Bae M A, Yamada K, Ijuin Y, et al. 1996. Aburatubolactam A, a novel inhibitor of superoxide anion generation from a marine microorganism. Heterocycl Commun, 2 (4) : 315–318 |
| Balk-Bindseil W, Helmke E, Weyland H, et al. 1995. Maremycin A and B, new diketopiperazines from a marine Streptomyces sp.. Liebigs Ann, (7) : 1291–1294 |
| Biabani M A F, Baake M, Lovisetto B, et al. 1998. Anthranilamides: new antimicroalgal active substances from a marine Streptomyces sp. . J Antibiot (Tokyo), 51 (3) : 333–340 DOI:10.7164/antibiotics.51.333 |
| Biabani M A F, Laatsh H, Helmke E, et al. 1997. δ-Indomycinone: a new member of pluramycin class of antibiotics isolated from marine Streptomyces sp. . J Antibiot (Tokyo), 50 (10) : 874–877 DOI:10.7164/antibiotics.50.874 |
| Boonlarppradab C, Kauffman C A, Jensen P R, et al. 2008. Marineosins A and B, cytotoxic spiroaminals from a marine-derived actinomycete. Org Lett, 10 (24) : 5505–5508 DOI:10.1021/ol8020644 |
| Bu Y Y, Yamazaki H, Ukai K, et al. 2014. Anti-mycobacterial nucleoside antibiotics from a marine-derived Streptomyces sp. TPU1236A. Mar Drugs, 12 (12) : 6102–6112 DOI:10.3390/md12126102 |
| Bugni T S, Woolery M, Kauffman C A, et al. 2006. Bohemamines from a marine-derived Streptomyces sp. . J Nat Prod, 69 (11) : 1626–1628 DOI:10.1021/np0602721 |
| Capon R J, Skene C, Lacey E, et al. 2000. Lorneamides A and B: two new aromatic amides from a southern Australian marine actinomycete. J Nat Prod, 63 (12) : 1682–1683 DOI:10.1021/np000241k |
| Carlson J C, Li S Y, Burr D A, et al. 2009. Isolation and characterization of tirandamycins from a marine-derived Streptomyces sp. . J Nat Prod, 72 (11) : 2076–2079 DOI:10.1021/np9005597 |
| Che Q, Li J, Li D H, et al. 2016. Structure and absolute configuration of drimentine I, an alkaloid from Streptomyces sp. CHQ-64. J Antibiot (Tokyo), 69 (6) : 467–469 DOI:10.1038/ja.2015.133 |
| Chen C X, Wang J, Guo H, et al. 2013. Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061. Appl Microbiol Biotechnol, 97 (9) : 3885–3892 DOI:10.1007/s00253-012-4681-0 |
| Chen G D, Gao H, Tang J S, et al. 2011. Benzamides and quinazolines from a mangrove actinomycetes Streptomyces sp. (No. 061316) and their inhibiting caspase-3 catalytic activity in vitro. Chem Pharm Bull, 59 (4) : 447–451 |
| Chen Y L, Zhang W J, Zhu Y G, et al. 2014. Elucidating hydroxylation and methylation steps tailoring piericidin A1 biosynthesis. Org Lett, 16 (3) : 736–739 DOI:10.1021/ol4034176 |
| Chen Z B, Hao J J, Wang L P, et al. 2016. New α-glucosidase inhibitors from marine algae-derived Streptomyces sp. OUCMDZ-3434. Sci Rep, 6 : 20004 |
| Cheng C, Othman E M, Reimer A, et al. 2016. Ageloline A, new antioxidant and antichlamydial quinolone from the marine sponge-derived bacterium Streptomyces sp. SBT345. Tetrahedron Lett, 57 (25) : 2786–2789 DOI:10.1016/j.tetlet.2016.05.042 |
| Cheng Y B, Jensen P R, Fenical W. 2013. Cytotoxic and antimicrobial napyradiomycins from two marine-derived, MAR 4 Streptomyces strains. Eur J Org Chem, (18) : 1751–3757 |
| Cho J Y, Kwon H C, Williams P G, et al. 2006a. Actinofuranones A and B, polyketides from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). J Nat Prod, 69 (3) : 425–428 DOI:10.1021/np050402q |
| Cho J Y, Kwon H C, Williams P G, et al. 2006b. Azamerone, a terpenoid phthalazinone from a marine-derived bacterium related to the genus Streptomyces (Actinomycetales). Org Lett, 8 (12) : 2471–2474 DOI:10.1021/ol060630r |
| Cho K W, Lee H S, Rho J R, et al. 2001. New lactone-containing metabolites from a marine-derived bacterium of the genus Streptomyces. J Nat Prod, 64 (5) : 664–667 DOI:10.1021/np000599g |
| Deng J J, Lu C H, Li S R, et al. 2014. p-terphenyl O-β-glucuronides, DNA topoisomerase inhibitors from Streptomyces sp. LZ35△gdmAI. Bioorg Med Chem Lett, 24 (5) : 1362–1365 DOI:10.1016/j.bmcl.2014.01.037 |
| Dickschat J S, Martens T, Brinkhoff T, et al. 2005. Volatiles released by a Streptomyces species isolated from the North Sea. Chem Biodivers, 2 (7) : 837–865 DOI:10.1002/(ISSN)1612-1880 |
| Ding L, Maier A, Fiebig H H, et al. 2011. Divergolides?A-D from a mangrove endophyte reveal an unparalleled plasticity in ansa-macrolide biosynthesis. Angew Chem Int Ed, 50 (7) : 1630–1634 DOI:10.1002/anie.201006165 |
| Ding L, Maier A, Fiebig H H, et al. 2012. Kandenols A-E, eudesmenes from an endophytic Streptomyces sp. of the mangrove tree Kandelia candel. J Nat Prod, 75 (12) : 2223–2227 |
| Ding L, Münch J, Goerls H, et al. 2010. Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorg Med Chem Lett, 20 (22) : 6685–6687 DOI:10.1016/j.bmcl.2010.09.010 |
| Ding L, Pfoh R, Rühl S, et al. 2009. T-muurolol sesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J Nat Prod, 72 (1) : 99–101 |
| Ding W J, Zhang S Q, Wang J H, et al. 2013. A new di-O-prenylated flavone from an actinomycete Streptomyces sp. MA-12. J Asian Nat Prod Res, 15 (2) : 209–214 DOI:10.1080/10286020.2012.751979 |
| El-Gendy M M A, Shaaban M, Shaaban K A, et al. 2008. Essramycin: a first triazolopyrimidine antibiotic isolated from nature. J Antibiot (Tokyo), 61 (3) : 149–157 DOI:10.1038/ja.2008.124 |
| Farnaes L, Coufal N G, Kauffman C A, et al. 2014. Napyradiomycin derivatives, produced by a marine-derived actinomycete, illustrate cytotoxicity by induction of apoptosis. J Nat Prod, 77 (1) : 15–21 DOI:10.1021/np400466j |
| Ford P W, Gadepalli M, Davidson B S. 1998. Halawanones A-D, new polycyclic quinones from a marine-derived streptomycete. J Nat Prod, 61 (10) : 1232–1236 DOI:10.1021/np980126y |
| Fremlin L, Farrugia M, Piggott A M, et al. 2011. Reveromycins revealed: new polyketide spiroketals from Australian marine-derived and terrestrial Streptomyces spp. A case of natural products vs. artifacts. Org Biomol Chem, 9 (4) : 1201–1211 |
| Fu P, Johnson M, Chen H, et al. 2014. Carpatamides A-C, cytotoxic arylamine derivatives from a marine-derived Streptomyces sp. . J Nat Prod, 77 (5) : 1245–1248 DOI:10.1021/np500207p |
| Fu P, Kong F D, Wang Y F, et al. 2013. Antibiotic metabolites from the coral-associated actinomycete Streptomyces sp. OUCMDZ-1703. Chin J Chem, 31 (1) : 100–104 |
| Fu P, La S, MacMillan J B. 2016. 1, 3-Oxazin-6-one derivatives and bohemamine-type pyrrolizidine alkaloids from a marine-derived Streptomyces spinoverrucosus. J Nat Prod, 79 (3) : 455–462 DOI:10.1021/acs.jnatprod.5b00604 |
| Fu P, Yang C L, Wang Y, et al. 2012a. Streptocarbazoles A and B, two novel indolocarbazoles from the marine-derived actinomycete strain Streptomyces sp. FMA. Org Lett, 14 (9) : 2422–2425 DOI:10.1021/ol3008638 |
| Fu P, Zhuang Y B, Wang Y, et al. 2012b. New indolocarbazoles from a mutant strain of the marine-derived actinomycete Streptomyces fradiae 007M135. Org Lett, 14 (24) : 6194–6197 DOI:10.1021/ol302940y |
| Fukuda T, Miller E D, Clark B R, et al. 2011. Structures and biosynthesis of the pyridinopyrones, polyenepyrones from a marine-derived Streptomyces species. J Nat Prod, 74 (8) : 1773–1778 DOI:10.1021/np200323e |
| Han Z, Xu Y, McConnell O, et al. 2012. Two antimycin A analogues from marine-derived actinomycete Streptomyces lusitanus. Mar Drugs, 10 (3) : 668–676 |
| Harinantenaina Rakotondraibe L, Rasolomampianina R, Park H Y, et al. 2015. Antiproliferative and antiplasmodial compounds from selected Streptomyces species. Bioorg Med Chem Lett, 25 (23) : 5646–5649 DOI:10.1016/j.bmcl.2015.07.103 |
| Harunari E, Imada C, Igarashi Y, et al. 2014. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. . Mar Drugs, 12 (1) : 491–507 DOI:10.3390/md12010491 |
| Hassan H M, Boonlarppradab C, Fenical W. 2016. Actinoquinolines A and B, anti-inflammatory quinoline alkaloids from a marine-derived Streptomyces sp. , strain CNP975. J Antibiot (Tokyo), 69 (7) : 511–514 DOI:10.1038/ja.2016.56 |
| Hawas U W, Shaaban M, Shaaban K A, et al. 2009. Mansouramycins A-D, cytotoxic isoquinolinequinones from a marine Streptomycete. J Nat Prod, 72 (12) : 2120–2124 DOI:10.1021/np900160g |
| Hayakawa Y, Shirasaki S, Shiba S, et al. 2007. Piericidins C7 and C8, new cytotoxic antibiotics produced by a marine Streptomyces sp. . J Antibiot, 60 (3) : 196–200 DOI:10.1038/ja.2007.22 |
| Hernandez I L C, Godinho M J L, Magalh?es A, et al. 2000. N-acetyl-γ-hydroxyvaline lactone, an unusual amino acid derivative from a marine streptomycete. J Nat Prod, 63 (5) : 664–665 DOI:10.1021/np990507r |
| Hohmann C, Schneider K, Bruntner C, et al. 2009. Albidopyrone, a new α-pyrone-containing metabolite from marine-derived Streptomyces sp. NTK 227. J Antibiot (Tokyo), 62 (2) : 75–79 DOI:10.1038/ja.2008.15 |
| Hosoya T, Hirokawa T, Takagi M, et al. 2012. Trichostatin analogues JBIR-109, JBIR-110, and JBIR-111 from the marine sponge-derived Streptomyces sp. RM72. J Nat Prod, 75 (2) : 285–289 DOI:10.1021/np200843k |
| Hu Y C, MacMillan J B. 2012. A new peptide isolated from a marine derived Streptomyces bacillaris. Nat Prod Commun, 7 (2) : 211–214 |
| Huang H, Cao Y, Tian L, et al. 2014. A new polyunsaturated acid from the marine-derived Streptomyces violans (No. HTTA-F04129). Chem Nat Compd, 50 (3) : 402–404 DOI:10.1007/s10600-014-0970-4 |
| Huang H B, Yang T T, Ren X M, et al. 2012. Cytotoxic angucycline class glycosides from the deep sea actinomycete Streptomyces lusitanus SCSIO LR32. J Nat Prod, 75 (2) : 202–208 DOI:10.1021/np2008335 |
| Huang X L, Gao Y, Xue D Q, et al. 2011. Streptomycindole, an indole alkaloid from a marine Streptomyces sp. DA22 associated with South China Sea sponge Craniella australiensis. Helv Chim Acta, 94 (10) : 1838–1842 |
| Huang Y F, Tian L, Fu H W, et al. 2006a. One new anthraquinone from marine Streptomyces sp. FX-58. Nat Prod Res, 20 (13) : 1207–1210 DOI:10.1080/14786410600899142 |
| Huang Y F, Tian L, Sun Y, et al. 2006b. Two new compounds from marine Streptomyces sp. FX-58. J Asian Nat Prod Res, 8 (6) : 495–498 DOI:10.1080/10286020500172285 |
| Hughes C C, Kauffman C A, Jensen P R, et al. 2010. Structures, reactivities, and antibiotic properties of the marinopyrroles A-F. J Org Chem, 75 (10) : 3240–3250 DOI:10.1021/jo1002054 |
| Hughes C C, Prieto-Davo A, Jensen P R, et al. 2008. The marinopyrroles, antibiotics of an unprecedented structure class from a marine Streptomyces sp. . Org Lett, 10 (4) : 629–631 DOI:10.1021/ol702952n |
| Igarashi Y, Asano D, Furihata K, et al. 2012. Absolute configuration of pterocidin, a potent inhibitor of tumor cell invasion from a marine-derived Streptomyces. Tetrahedron Lett, 53 (6) : 654–656 DOI:10.1016/j.tetlet.2011.11.115 |
| Igarashi Y, Shimasaki R, Miyanaga S, et al. 2010. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp. . J Antibiot (Tokyo), 63 (9) : 563–565 DOI:10.1038/ja.2010.70 |
| Igarashi Y, Zhou T, Sato S, et al. 2013. Akaeolide, a carbocyclic polyketide from marine-derived Streptomyces. Org Lett, 15 (22) : 5678–5681 DOI:10.1021/ol402661r |
| Imamura N, Nishijima M, Adachi K, et al. 1993. Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J Antibiot (Tokyo), 46 (2) : 241–246 DOI:10.7164/antibiotics.46.241 |
| Itoh T, Kinoshita M, Aoki S, et al. 2003. Komodoquinone A, a novel neuritogenic anthracycline, from marine Streptomyces sp. KS3. J Nat Prod, 66 (10) : 1373–1377 DOI:10.1021/np030212k |
| Ivanova V, Oriol M, Montes M J, et al. 2001. Secondary metabolites from a Streptomyces strain isolated from Livingston Island, Antarctica. Z Naturforsch C, 56 (1-2) : 1–5 DOI:10.1515/znc-2001-1-201 |
| Iwata F, Sato S, Mukai T, et al. 2009. Lorneic acids, trialkyl-substituted aromatic acids from a marine-derived actinomycete. J Nat Prod, 72 (11) : 2046–2048 DOI:10.1021/np900353y |
| Izumikawa M, Kawahara T, Hwang J H, et al. 2013. JBIR-107, a new metabolite from the marine-sponge-derived actinomycete, Streptomyces tateyamensis NBRC 105047. Biosci Biotechnol Biochem, 77 (3) : 663–665 DOI:10.1271/bbb.120832 |
| Izumikawa M, Khan S T, Komaki H, et al. 2010a. JBIR-31, a new teleocidin analog, produced by salt-requiring Streptomyces sp. NBRC 105896 isolated from a marine sponge. J Antibiot (Tokyo), 63 (1) : 33–36 |
| Izumikawa M, Khan S T, Takagi M, et al. 2010b. Sponge-derived Streptomyces producing isoprenoids via the mevalonate pathway. J Nat Prod, 73 (2) : 208–212 DOI:10.1021/np900747t |
| Jang K H, Nam S J, Locke J B, et al. 2013. Anthracimycin, a potent anthrax antibiotic from a marine-derived actinomycete. Angew Chem Int Ed, 52 (30) : 7822–7824 DOI:10.1002/anie.v52.30 |
| Jeong S Y, Shin H J, Kim T S, et al. 2006. Streptokordin, a new cytotoxic compound of the methylpyridine class from a marine-derived Streptomyces sp. KORDI-3238. J Antibiot (Tokyo), 59 (4) : 234–240 |
| Jiang Z D, Jensen P R, Fenical W. 1997. Actinoflavoside, a novel flavonoid-like glycoside produced by a marine bacterium of the genus Streptomyces. Tetrahedron Lett, 38 (29) : 5065 DOI:10.1016/S0040-4039(97)01127-1 |
| Kaysser L, Bernhardt P, Nam S J, et al. 2012. Merochlorins A-D, cyclic meroterpenoid antibiotics biosynthesized in divergent pathways with vanadium-dependent chloroperoxidases. J Am Chem Soc, 134 (29) : 11988–11991 DOI:10.1021/ja305665f |
| Kim D G, Moon K, Kim S H, et al. 2012. Bahamaolides A and B, antifungal polyene polyol macrolides from the marine actinomycete Streptomyces sp. . J Nat Prod, 75 (5) : 959–967 DOI:10.1021/np3001915 |
| Kim H, Yang I, Patil R S, et al. 2014. Anithiactins A-C, modified 2-phenylthiazoles from a mudflat-derived Streptomyces sp. . J Nat Prod, 77 (12) : 2716–2719 DOI:10.1021/np500558b |
| Ko K, Lee S H, Kim S H, et al. 2014. Lajollamycins, nitro group-bearing spiro-β-lactone-γ-lactams obtained from a marine-derived Streptomyces sp. . J Nat Prod, 77 (9) : 2099–2104 DOI:10.1021/np500500t |
| Kock I, Maskey R P, Biabani M A F, et al. 2005. 1-Hydroxy-1-norresistomycin and resistoflavin methyl ether: new antibiotics from marine-derived streptomycetes. J Antibiot (Tokyo), 58 (8) : 530–534 DOI:10.1038/ja.2005.73 |
| Kondratyuk T P, Park E J, Yu R, et al. 2012. Novel marine phenazines as potential cancer chemopreventive and anti-inflammatory agents. Mar Drugs, 10 (2) : 451–464 |
| Kunz A L, Labes A, Wiese J, et al. 2014. Nature's lab for derivatization: new and revised structures of a variety of streptophenazines produced by a sponge-derived Streptomyces strain. Mar Drugs, 12 (4) : 1699–1714 DOI:10.3390/md12041699 |
| Kwon H C, Espindola A P D M, Park J S, et al. 2010. Nitropyrrolins A-E, cytotoxic farnesyl-α-nitropyrroles from a marine-derived bacterium within the actinomycete family Streptomycetaceae. J Nat Prod, 73 (12) : 2047–2052 DOI:10.1021/np1006229 |
| Lane A L, Nam S J, Fukuda T, et al. 2013. Structures and comparative characterization of biosynthetic gene clusters for cyanosporasides, enediyne-derived natural products from marine actinomycetes. J Am Chem Soc, 135 (11) : 4171–4174 DOI:10.1021/ja311065v |
| Le T C, Yang I, Yoon Y J, et al. 2016. Ansalactams B-D illustrate further biosynthetic plasticity within the ansamycin pathway. Org Lett, 18 (9) : 2256–2259 DOI:10.1021/acs.orglett.6b00892 |
| Lee H K, Lee D S, Lim J, et al. 1998. Topoisomerase I inhibitors from the Streptomyces sp. strain KM86-9B isolated from a marine sponge. Arch Pharm Res, 21 (6) : 729–733 |
| Lee H S, Shin H J, Jang K H, et al. 2005. Cyclic peptides of the nocardamine class from a marine-derived bacterium of the genus Streptomyces. J Nat Prod, 68 (4) : 623–625 DOI:10.1021/np040220g |
| Lee J, Kim H, Lee T G, et al. 2014. Anmindenols A and B, inducible nitric oxide synthase inhibitors from a marine-derived Streptomyces sp. . J Nat Prod, 77 (6) : 1528–1531 DOI:10.1021/np500285a |
| Li B, Chen G, Bai J, et al. 2011a. A bisamide and four diketopiperazines from a marine-derived Streptomyces sp. . J Asian Nat Prod Res, 13 (12) : 1146–1150 DOI:10.1080/10286020.2011.617744 |
| Li F C, Maskey R P, Qin S, et al. 2005. Chinikomycins A and B: isolation, structure elucidation, and biological activity of novel antibiotics from a marine Streptomyces sp. isolate M045#. J Nat Prod, 68 (3) : 349–353 |
| Li J, Lu C H, Zhao B B, et al. 2008. Phaeochromycins F-H, three new polyketide metabolites from Streptomyces sp. DSS-18. Beilstein J Org Chem, 4 (1) : 46 |
| Li K, Li Q L, Ji N Y, et al. 2011b. Deoxyuridines from the marine sponge associated actinomycete Streptomyces microflavus. Mar Drugs, 9 (5) : 690–695 |
| Lian X Y, Zhang Z Z. 2013. Indanomycin-related antibiotics from marine Streptomyces antibioticus PTZ0016. Nat Prod Res, 27 (23) : 2161–2167 DOI:10.1080/14786419.2013.793688 |
| Liang Y, Chen L, Ye X W, et al. 2016a. New streptophenazines from marine Streptomyces sp. 182SMLY. Nat Prod Res, : 1–7 |
| Liang Y, Xie X, Chen L, et al. 2016b. Bioactive polycyclic quinones from marine Streptomyces sp. 182SMLY. Mar Drugs, 14 (1) : 1–11 |
| Lin Z J, Antemano R R, Hughen R W, et al. 2010. Pulicatins A-E, neuroactive thiazoline metabolites from cone snail-associated bacteria. J Nat Prod, 73 (11) : 1922–1926 DOI:10.1021/np100588c |
| Lin Z J, Flores M, Forteza I, et al. 2012. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. . J Nat Prod, 75 (4) : 644–649 DOI:10.1021/np200886x |
| Lin Z J, Koch M, Pond C D, et al. 2014. Structure and activity of lobophorins from a turrid mollusk-associated Streptomyces sp. . J Antibiot (Tokyo), 67 (1) : 121–126 DOI:10.1038/ja.2013.115 |
| Lin Z J, Reilly C A, Antemano R, et al. 2011. Nobilamides A-H, long-acting transient receptor potential vanilloid-1 (TRPV1) antagonists from mollusk-associated bacteria. J Med Chem, 54 (11) : 3746–3755 DOI:10.1021/jm101621u |
| Liu D, Yang A G, Wu C M, et al. 2014. Lipid-?lowering effects of farnesylquinone and related analogues from the marine-?derived Streptomyces nitrosporeus. Bioorg Med Chem Lett, 24 (22) : 5288–5293 DOI:10.1016/j.bmcl.2014.09.049 |
| Liu N, Song F Y, Shang F, et al. 2015. Mycemycins A-E, new dibenzoxazepinones isolated from two different Streptomycetes. Mar Drugs, 13 (10) : 6247–6258 DOI:10.3390/md13106247 |
| Lorente A, Pla D, Ca?edo L M, et al. 2010. Isolation, structural assignment, and total synthesis of barmumycin. J Org Chem, 75 (24) : 8508–8515 DOI:10.1021/jo101834c |
| Luo M H, Tang G L, Ju J H, et al. 2016. A new diketopiperazine derivative from a deep sea-derived Streptomyces sp. SCSIO 04496. Nat Prod Res, 30 (2) : 138–143 DOI:10.1080/14786419.2015.1045509 |
| Macherla V R, Liu J, Bellows C, et al. 2005. Glaciapyrroles A, B, and C, pyrrolosesquiterpenes from a Streptomyces sp. isolated from an Alaskan marine sediment. J Nat Prod, 68 (5) : 780–783 |
| Mahyudin N A, Blunt J W, Cole A L J, et al. 2012. The isolation of a new S-methyl benzothioate compound from a marine-derived Streptomyces sp. . J Biomed Biotechnol, 2012 : 894708 |
| Manam R R, Teisan S, White D J, et al. 2005. Lajollamycin, a nitro-tetraene spiro-β-lactone-γ-lactam antibiotic from the marine actinomycete Streptomyces nodosus. J Nat Prod, 68 (2) : 240–243 DOI:10.1021/np049725x |
| Martin G D A, Tan L T, Jensen P R, et al. 2007. Marmycins A and B, cytotoxic pentacyclic C-glycosides from a marine sediment-derived actinomycete related to the genus Streptomyces. J Nat Prod, 70 (9) : 1406–1409 DOI:10.1021/np060621r |
| Maskey R P, Helmke E, Fiebig H H, et al. 2002. Parimycin: isolation and structure elucidation of a novel cytotoxic 2, 3-dihydroquinizarin analogue of γ-indomycinone from a marine Streptomycete isolate. J Antibiot (Tokyo), 55 (12) : 1031–1035 DOI:10.7164/antibiotics.55.1031 |
| Maskey R P, Sevvana M, Usón I, et al. 2004. Gutingimycin: a highly complex metabolite from a marine streptomycete. Angew Chem Int Ed, 43 (10) : 1281–1283 DOI:10.1002/(ISSN)1521-3773 |
| Matsuo Y, Kanoh K, Jang J H, et al. 2011. Streptobactin, a tricatechol-type siderophore from marine-derived Streptomyces sp. YM5-799. J Nat Prod, 74 (11) : 2371–2376 DOI:10.1021/np200290j |
| Miller E D, Kauffman C A, Jensen P R, et al. 2007. Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus Streptomyces. J Org Chem, 72 (2) : 323–330 DOI:10.1021/jo061064g |
| Mitchell S S, Nicholson B, Teisan S, et al. 2004. Aureoverticillactam, a novel 22-atom macrocyclic lactam from the marine actinomycete Streptomyces aureoverticillatus. J Nat Prod, 67 (8) : 1400–1402 DOI:10.1021/np049970g |
| Mitova M I, Lang G, Wiese J, et al. 2008. Subinhibitory concentrations of antibiotics induce phenazine production in a marine Streptomyces sp. . J Nat Prod, 71 (5) : 824–827 DOI:10.1021/np800032a |
| Moon K, Ahn C H, Shin Y, et al. 2014. New benzoxazine secondary metabolites from an arctic actinomycete. Mar Drugs, 12 (5) : 2526–2538 DOI:10.3390/md12052526 |
| Motohashi K, Inaba K, Fuse S, et al. 2011. JBIR-56 and JBIR-57, 2 (1H)-pyrazinones from a marine sponge-derived Streptomyces sp. SpD081030SC-03. J Nat Prod, 74 (7) : 1630–1635 DOI:10.1021/np200386c |
| Motohashi K, Irie K, Toda T, et al. 2008. 5-Dimethylallylindole-3-carboxylic acid and A80915G-8-acid produced by marine-derived Streptomyces sp. MS239. J Antibiot, 61 (2) : 75–80 DOI:10.1038/ja.2008.113 |
| Motohashi K, Takagi M, Shin-Ya K. 2010a. Tetracenoquinocin and 5-iminoaranciamycin from a sponge-derived Streptomyces sp. Sp080513GE-26. J Nat Prod, 73 (4) : 755–758 DOI:10.1021/np9007409 |
| Motohashi K, Takagi M, Shin-Ya K. 2010b. Tetrapeptides possessing a unique skeleton, JBIR-34 and JBIR-35, isolated from a sponge-derived actinomycete, Streptomyces sp. Sp080513GE-23. J Nat Prod, 73 (2) : 226–228 DOI:10.1021/np900810r |
| Motohashi K, Toda T, Sue M, et al. 2010c. Isolation and structure elucidation of tumescenamides A and B, two peptides produced by Streptomyces tumescens YM23-260. J Antibiot (Tokyo), 63 (9) : 549–552 DOI:10.1038/ja.2010.73 |
| Mukku V J R V, Speitling M, Laatsch H, et al. 2000. New butenolides from two marine streptomycetes. J Nat Prod, 63 (11) : 1570–1572 DOI:10.1021/np0001676 |
| Mullowney M W, ó hAinmhire E, Shaikh A, et al. 2014. Diazaquinomycins E-G, novel diaza-anthracene analogs from a marine-derived Streptomyces sp. . Mar Drugs, 12 (6) : 3574–3586 DOI:10.3390/md12063574 |
| Na M, Meujo D A F, Kevin D, et al. 2008. A new antimalarial polyether from a marine Streptomyces sp. H668. Tetrahedron Lett, 49 (44) : 6282–6285 DOI:10.1016/j.tetlet.2008.08.052 |
| Nachtigall J, Schneider K, Bruntner C, et al. 2011. Benzoxacystol, a benzoxazine-type enzyme inhibitor from the deep-sea strain Streptomyces sp. NTK 935. J Antibiot (Tokyo), 64 (6) : 453–457 DOI:10.1038/ja.2011.26 |
| Nakamura H, Iitaka Y, Kitahara T, et al. 1977. Structure of Aplasmomycin. J Antibiot (Tokyo), 30 (9) : 714–719 DOI:10.7164/antibiotics.30.714 |
| Nam S J, Kauffman C A, Jensen P R, et al. 2011. Isolation and characterization of actinoramides A—C, highly modified peptides from a marine Streptomyces sp. . Tetrahedron, 67 (35) : 6707–6712 DOI:10.1016/j.tet.2011.04.051 |
| Nam S J, Kauffman C A, Paul L A, et al. 2013. Actinoranone, a cytotoxic meroterpenoid of unprecedented structure from a marine adapted Streptomyces sp. . Org Lett, 15 (21) : 5400–5403 DOI:10.1021/ol402080s |
| Nong X H, Zhang X Y, Xu X Y, et al. 2016. Nahuoic acids B?E, polyhydroxy polyketides from the marine-derived Streptomyces sp. SCSGAA. J Nat Prod, 79 (1) : 141–148 DOI:10.1021/acs.jnatprod.5b00805 |
| Okami Y, Hotta K, Yoshida M, et al. 1979. New aminoglycoside antibiotics, istamycins A and B. J Antibiot (Tokyo), 32 (9) : 964–966 DOI:10.7164/antibiotics.32.964 |
| Okami Y, Okazaki T, Kitahara T, et al. 1976. Studies on marine microorganisms. V. A new antibiotic, aplasmomycin, produced by a streptomycete isolated from shallow sea mud. J Antibiot (Tokyo), 29 (10) : 1019–1025 |
| Pan E D, Jamison M, Yousufuddin M, et al. 2012. Ammosamide D, an oxidatively ring opened ammosamide analog from a marine-derived Streptomyces variabilis. Org Lett, 14 (9) : 2390–2393 DOI:10.1021/ol300806e |
| Pan H Q, Zhang S Y, Wang N, et al. 2013. New spirotetronate antibiotics, lobophorins H and I, from a South China Sea-derived Streptomyces sp. 12A35. Mar Drugs, 11 (10) : 3891–3901 DOI:10.3390/md11103891 |
| Pathirana C, Jensen P R, Dwight R, et al. 1992. Rare phenazine L-quinovose esters from a marine actinomycete. J Org Chem, 57 (2) : 740–742 DOI:10.1021/jo00028a060 |
| Pérez M, Crespo C, Schleissner C, et al. 2009. Tartrolon D, a cytotoxic macrodiolide from the marine-derived actinomycete Streptomyces sp. MDG-04-17-069. J Nat Prod, 72 (12) : 2192–2194 |
| Pérez M, Schleissner C, Fernández R, et al. 2016. PM100117 and PM100118, new antitumor macrolides produced by a marine Streptomyces caniferus GUA-06-05-006A. J Antibiot (Tokyo), 69 (5) : 388–394 DOI:10.1038/ja.2015.121 |
| Phipps R K, Blunt J W, Cole A L J, et al. 2004. Anthracycline derivatives from a marine-derived New Zealand Streptomycete. Arkivoc, 2004 (10) : 94–100 DOI:10.3998/ark.5550190.0005.a10 |
| Pimentel-Elardo S M, Buback V, Gulder T A M, et al. 2011. New tetromycin derivatives with anti-trypanosomal and protease inhibitory activities. Mar Drugs, 9 (10) : 1682–1697 |
| Poumale H M P, Ngadjui B T, Helmke E, et al. 2006. New anthraquinones from a marine Streptomyces sp. -isolation, structure determination and biological activities. Z Naturforsch B, 61 (11) : 1450–1454 |
| Pusecker K, Laatsch H, Helmke E, et al. 1997. Dihydrophencomycin methyl ester, a new phenazine derivative from a marine Streptomycete. J Antibiot (Tokyo), 50 (6) : 479–483 DOI:10.7164/antibiotics.50.479 |
| Quitschau M, Schuhmann T, Piel J, et al. 2008. The new metabolite (S)-cinnamoylphosphoramide from Streptomyces sp. and its total synthesis. Eur J Org Chem, (30) : 5117–5124 |
| Raju R, Khalil Z G, Piggott A M, et al. 2014. Mollemycin A: an antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB-M0244). Org Lett, 16 (6) : 1716–1719 DOI:10.1021/ol5003913 |
| Raju R, Piggott A M, Barrientos Diaz L X, et al. 2010a. Heronapyrroles A-C: farnesylated 2-nitropyrroles from an Australian marine-derived Streptomyces sp. . Org Lett, 12 (22) : 5158–5161 DOI:10.1021/ol102162d |
| Raju R, Piggott A M, Conte M M, et al. 2010b. Heronamides A-C, new polyketide macrolactams from an Australian marine-derived Streptomyces sp. A biosynthetic case for synchronized tandem electrocyclization. Org Biomol Chem, 8 (20) : 4682–4689 |
| Raju R, Piggott A M, Conte M M, et al. 2009. Naseseazines A and B: a new dimeric diketopiperazine framework from a marine-derived actinomycete, Streptomyces sp. . Org Lett, 11 (17) : 3862–3865 DOI:10.1021/ol901466r |
| Raju R, Piggott A M, Khalil Z, et al. 2012. Heronamycin A: a new benzothiazine ansamycin from an Australian marine-derived Streptomyces sp. . Tetrahedron Lett, 53 (9) : 1063–1065 DOI:10.1016/j.tetlet.2011.12.064 |
| Salem S M, Kancharla P, Florova G, et al. 2014. Elucidation of final steps of the marineosins biosynthetic pathway through identification and characterization of the corresponding gene cluster. J Am Chem Soc, 136 (12) : 4565–4574 DOI:10.1021/ja411544w |
| Jose S L, Marta M I, Julia P B, et al. 2003. New cytotoxic indolic metabolites from a marine Streptomyces. J Nat Prod, 66 (6) : 863–864 DOI:10.1021/np0204444 |
| Sato S, Iwata F, Mukai T, et al. 2009. Indoxamycins A-F. Cytotoxic tricycklic polypropionates from a marine-derived actinomycete. J Org Chem, 74 (15) : 5502–5509 |
| Sato S, Iwata F, Yamada S, et al. 2011. Usabamycins A-C: new anthramycin-type analogues from a marine-derived actinomycete. Bioorg Med Chem Lett, 21 (23) : 7099–7101 DOI:10.1016/j.bmcl.2011.09.086 |
| Schleissner C, Pérez M, Losada A, et al. 2011. Antitumor actinopyranones produced by Streptomyces albus POR-04-15-053 isolated from a marine sediment. J Nat Prod, 74 (7) : 1590–1596 DOI:10.1021/np200196j |
| Schneemann I, Kajahn I, Ohlendorf B, et al. 2010. Mayamycin, a cytotoxic polyketide from a Streptomyces strain isolated from the marine sponge Halichondria panicea. J Nat Prod, 73 (7) : 1309–1312 DOI:10.1021/np100135b |
| Schumacher R W, Talmage S C, Miller S A, et al. 2003. Isolation and structure determination of an antimicrobial ester from a marine sediment-derived bacterium. J Nat Prod, 66 (9) : 1291–1293 DOI:10.1021/np020594e |
| Shaaban K A, Helmke E, Kelter G, et al. 2011. Glucopiericidin C: a cytotoxic piericidin glucoside antibiotic produced by a marine Streptomyces isolate. J Antibiot (Tokyo), 64 (2) : 205–209 DOI:10.1038/ja.2010.125 |
| Shaaban K A, Shaaban M, Facey P, et al. 2008. Electrospray ionization mass spectra of piperazimycins A and B and gamma-butyrolactones from a marine-derived Streptomyces sp. . J Antibiot (Tokyo), 61 (12) : 736–746 DOI:10.1038/ja.2008.87 |
| Shin B, Ahn S, Noh M, et al. 2015. Suncheonosides A?D, benzothioate glycosides from a marine-derived Streptomyces sp. . J Nat Prod, 78 (6) : 1390–1396 DOI:10.1021/acs.jnatprod.5b00284 |
| Shin H J, Lee H S, Lee J S, et al. 2014. Violapyrones H and I, new cytotoxic compounds isolated from Streptomyces sp. associated with the marine starfish Acanthaster planci. Mar Drugs, 12 (6) : 3283–3291 |
| Sitachitta N, Gadepalli M, Davidson B S. 1996. New α-pyrone-containing metabolites from a marine-derived actinomycete. Tetrahedron, 52 (24) : 8073–8080 DOI:10.1016/0040-4020(96)00391-2 |
| Socha A M, Garcia D, Sheffer R, et al. 2006. Antibiotic bisanthraquinones produced by a Streptomycete isolated from a Cyanobacterium. J Nat Prod, 69 (7) : 1070–1073 DOI:10.1021/np050449b |
| Song Y X, Huang H B, Chen Y C, et al. 2013. Cytotoxic and antibacterial marfuraquinocins from the deep south china sea-derived Streptomyces niveus SCSIO 3406. J Nat Prod, 76 (12) : 2263–2268 DOI:10.1021/np4006025 |
| Song Y X, Li Q L, Liu X, et al. 2014. Cyclic hexapeptides from the deep south china sea-derived Streptomyces scopuliridis SCSIO ZJ46 active against pathogenic gram-positive bacteria. J Nat Prod, 77 (8) : 1937–1941 DOI:10.1021/np500399v |
| Song Y X, Liu G F, Li J, et al. 2015. Cytotoxic and antibacterial angucycline-and prodigiosin-analogues from the deep-sea derived Streptomyces sp. SCSIO 11594. Mar Drugs, 13 (3) : 1304–1316 DOI:10.3390/md13031304 |
| Strangman W K, Kwon H C, Broide D, et al. 2009. Potent inhibitors of pro-inflammatory cytokine production produced by a marine-derived bacterium. J Med Chem, 52 (8) : 2317–2327 DOI:10.1021/jm801110j |
| Stritzke K, Schulz S, Laatsch H, et al. 2004. Novel caprolactones from a marine streptomycete. J Nat Prod, 67 (3) : 395–401 DOI:10.1021/np030321z |
| Sugiyama R, Nishimura S, Matsumori N, et al. 2014. Structure and biological activity of 8-deoxyheronamide C from a marine-derived Streptomyces sp. : heronamides target saturated hydrocarbon chains in lipid membranes. J Am Chem Soc, 136 (14) : 5209–5212 |
| Sun D D, Sun W, Yu Y X, et al. 2014a. A new glutarimide derivative from marine sponge-derived Streptomyces anulatus S71. Nat Prod Res, 28 (19) : 1602–1606 DOI:10.1080/14786419.2014.928877 |
| Sun P, Maloney K N, Nam S J, et al. 2011. Fijimycins A-C, three antibacterial etamycin-class depsipeptides from a marine-derived Streptomyces sp. . Bioorg Med Chem, 19 (22) : 6557–6562 DOI:10.1016/j.bmc.2011.06.053 |
| Sun Y, Takada K, Nogi Y, et al. 2014b. Lower homologues of ahpatinin, aspartic protease inhibitors, from a marine Streptomyces sp. . J Nat Prod, 77 (7) : 1749–1752 DOI:10.1021/np500337m |
| Supong K, Thawai C, Suwanborirux K, et al. 2012. Antimalarial and antitubercular C-glycosylated benz[α]anthraquinones from the marine-derived Streptomyces sp. BCC45596. Phytochem Lett, 5 (3) : 651–656 DOI:10.1016/j.phytol.2012.06.015 |
| Takada K, Ninomiya A, Naruse M, et al. 2013. Surugamides A-E, cyclic octapeptides with four D-amino acid residues, from a marine Streptomyces sp. LC?MS-aided inspection of partial hydrolysates for the distinction of D-and L-amino acid residues in the sequence. J Org Chem, 78 (13) : 6746–6750 |
| Takahashi C, Takada T, Yamada T, et al. 1994. Halichomycin, a new class of potent cytotoxic macrolide produced by an actinomycete from a marine fish. Tetrahedron Lett, 35 (28) : 5013–5014 DOI:10.1016/S0040-4039(00)73307-7 |
| Tapiolas D M, Roman M, Fenical W, et al. 1991. Oc talactins A and B: cytotoxic eight-membered-ring lactones from a marine bacterium, Streptomyces sp. . J Am Chem Soc, 113 (12) : 4682–4683 DOI:10.1021/ja00012a048 |
| Trischman J A, Tapiolas D M, Jensen P R, et al. 1994. Salinamides A and B: anti-inflammatory depsipeptides from a marine Streptomycete. J Am Chem Soc, 116 (2) : 757–758 DOI:10.1021/ja00081a042 |
| Ueda J Y, Khan S T, Takagi M, et al. 2010. JBIR-58, a new salicylamide derivative, isolated from a marine sponge-derived Streptomyces sp. SpD081030ME-02. J Antibiot (Tokyo), 63 (5) : 267–269 DOI:10.1038/ja.2010.26 |
| Um S, Choi T J, Kim H, et al. 2013a. Ohmyungsamycins A and B: cytotoxic and antimicrobial cyclic peptides produced by Streptomyces sp. from a volcanic island. J Org Chem, 78 (24) : 12321–12329 |
| Um S, Kim Y J, Kwon H, et al. 2013b. Sungsanpin, a lasso peptide from a deep-sea streptomycete. J Nat Prod, 76 (5) : 873–879 DOI:10.1021/np300902g |
| Vicente J, Stewart A K, Wagoner R M, et al. 2015. Monacyclinones, new angucyclinone metabolites isolated from Streptomyces sp. m7_15 associated with the puertorican sponge Scopalina ruetzleri. Mar Drugs, 13 (8) : 4682–4700 |
| Wang F F, Xu M J, Li Q S, et al. 2010. p-Aminoacetophenonic acids produced by a mangrove endophyte Streptomyces sp. (strain HK10552). Molecules, 15 (4) : 2782–2790 DOI:10.3390/molecules15042782 |
| Wang P, Xi L J, Liu P P, et al. 2013. Diketopiperazine derivatives from the marine-derived actinomycete Streptomyces sp. FXJ7.328. Mar Drugs, 11 (4) : 1035–1049 DOI:10.3390/md11041035 |
| Wei R B, Xi T, Li J, et al. 2011. Lobophorin C and D, new kijanimicin derivatives from a marine sponge-associated actinomycetal strain AZS17. Mar Drugs, 9 (3) : 359–368 |
| Williams D E, Dalisay D S, Li F L, et al. 2013. Nahuoic acid A produced by a Streptomyces sp. isolated from a marine sediment is a selective SAM-competitive inhibitor of the histone methyltransferase SETD8. Org Lett, 15 (2) : 414–417 |
| Williams D E, Dalisay D S, Patrick B O, et al. 2011. Padanamides A and B, highly modified linear tetrapeptides produced in culture by a Streptomyces sp. isolated from a marine sediment. Org Lett, 13 (15) : 3936–3939 |
| Williams D E, Izard F, Arnould S, et al. 2016. Structures of nahuoic acids B?E produced in culture by a Streptomyces sp. isolated from a marine sediment and evidence for the inhibition of the histone methyl transferase SETD8 in human cancer cells by nahuoic acid A. J Org Chem, 81 (4) : 1324–1332 |
| Wilson M C, Nam S J, Gulder T A M, et al. 2011. Structure and biosynthesis of the marine streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit. J Am Chem Soc, 133 (6) : 1971–1977 DOI:10.1021/ja109226s |
| Wu C Y, Tan Y, Gan M L, et al. 2013a. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using pcr-based screening. J Nat Prod, 76 (11) : 2153–2157 |
| Wu S J, Fotso S, Li F C, et al. 2006. N-carboxamido-staurosporine and selina-4(14), 7(11)-diene-8, 9-diol, new metabolites from a marine Streptomyces sp. . J Antibiot (Tokyo), 59 (6) : 331–337 DOI:10.1038/ja.2006.46 |
| Wu S J, Fotso S, Li F C, et al. 2007. Amorphane sesquiterpenes from a marine Streptomyces sp. . J Nat Prod, 70 (2) : 304–306 DOI:10.1021/np050358e |
| Wu Z C, Li S M, Li J, et al. 2013b. Antibacterial and cytotoxic new napyradiomycins from the marine-derived Streptomyces sp. SCSIO 10428. Mar Drugs, 11 (6) : 2113–2125 DOI:10.3390/md11062113 |
| Xie X C, Mei W L, Zhao Y X, et al. 2006. A new degraded sesquiterpene from marine actinomycete streptomyces. 0616208. Chinese Chem Lett, 17 (11) : 1463–1465 |
| Xie Z P, Liu B, Wang H P, et al. 2012. Kiamycin, a unique cytotoxic angucyclinone derivative from a marine Streptomyces sp. . Mar Drugs, 10 (3) : 551–558 |
| Xu L Y, Quan X S, Wang C, et al. 2011. Antimycins A19 and A20, two new antimycins produced by marine actinomycete Streptomyces antibioticus H74-18. J Antibiot (Tokyo), 64 (10) : 661–665 DOI:10.1038/ja.2011.65 |
| Xu M J, Liu X J, Zhao Y L, et al. 2012. Identification and characterization of an anti-fibrotic benzopyran compound isolated from mangrove-derived Streptomyces xiamenensis. Mar Drugs, 10 (3) : 639–654 |
| Xu X L, Yin L Y, Wang S Y, et al. 2013. Cycloheximide acid A, a new cycloheximide derivative from marine derived Streptomyces sp. from East China Sea. Rec Nat Prod, 7 (4) : 292–295 |
| Xu Z L, Baunach M, Ding L, et al. 2014. Biosynthetic code for divergolide assembly in a bacterial mangrove endophyte. Chembiochem, 15 (9) : 1274–1279 DOI:10.1002/cbic.v15.9 |
| Yang A G, Si L L, Shi Z P, et al. 2013a. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the Arctic Streptomyces nitrosporeus. Org Lett, 15 (20) : 5366–5369 DOI:10.1021/ol4026809 |
| Yang X W, Peng K, Liu Z, et al. 2013b. Strepsesquitriol, a rearranged zizaane-type sesquiterpenoid from the deep-sea-derived actinomycete Streptomyces sp. SCSIO 10355. J Nat Prod, 76 (12) : 2360–2363 DOI:10.1021/np400923c |
| Yao C B F, Schiebel M, Helmke E, et al. 2006. Prefluostatin and new urauchimycin derivatives produced by Streptomycete isolates. Z Naturforsch B, 61 (3) : 320–325 |
| Yixizhuoma, Tsukahara K, Toume K, et al. 2015. Novel cytotoxic isobenzofuran derivatives from Streptomyces sp. IFM 11490. Tetrahedron Lett, 56 (46) : 6345–6347 DOI:10.1016/j.tetlet.2015.09.116 |
| Yuan G J, Hong K, Lin H P, et al. 2013. New azalomycin F analogs from mangrove Streptomyces sp. 211726 with activity against microbes and cancer cells. Mar Drugs, 11 (3) : 817–829 |
| Yuan G J, Lin H P, Wang C, et al. 2011. 1H and 13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of Azalomycins F3a, F4a and F5a. Magn Reson Chem, 49 (1) : 30–37 |
| Zafrir Ilan E, Torres M R, Prudhomme J, et al. 2013. Farnesides A and B, sesquiterpenoid nucleoside ethers from a marine-derived Streptomyces sp. , strain CNT-372 from Fiji. J Nat Prod, 76 (9) : 1815–1818 |
| Zhang H Y, Wang H P, Cui H L, et al. 2011. A new anthracene derivative from marine Streptomyces sp. W007 exhibiting highly and selectively cytotoxic activities. Mar Drugs, 9 (9) : 1502–1509 |
| Zhang J L, Qian Z Y, Wu X K, et al. 2014a. Juanlimycins A and B, ansamycin macrodilactams from Streptomyces sp. . Org Lett, 16 (10) : 2752–2755 DOI:10.1021/ol501072t |
| Zhang Q B, Mándi A, Li S M, et al. 2012a. N-N-Coupled indolo-sesquiterpene atropo-diastereomers from a marine-derived actinomycete. Eur J Org Chem, (27) : 5256–5262 |
| Zhang W J, Ma L, Li S M, et al. 2014b. Indimicins A-E, bisindole alkaloids from the deep-sea-derived Streptomyces sp. SCSIO 03032. J Nat Prod, 77 (8) : 1887–1892 DOI:10.1021/np500362p |
| Zhang W J, Li S M, Zhu Y G, et al. 2014c. Heronamides D?F, polyketide macrolactams from the deep-sea-derived Streptomyces sp. SCSIO 03032. J Nat Prod, 77 (2) : 388–391 DOI:10.1021/np400665a |
| Zhang W J, Liu Z, Li S M, et al. 2012b. Spiroindimicins A-D: new bisindole alkaloids from a deep-sea-derived actinomycete. Org Lett, 14 (13) : 3364–3367 DOI:10.1021/ol301343n |
| Zhang Y, Zhou X, Huang H B, et al. 2013. 03219A, a new Δ8, 9-pregnene isolated from Streptomyces sp. SCSIO 03219 obtained from a South China Sea sediment. J Antibiot (Tokyo), 66 (6) : 327–331 |
| Zhang Y M, Li H Y, Hu C, et al. 2016. Ergosterols from the culture broth of marine Streptomyces anandii H41-59. Mar Drugs, 14 (5) |
| Zhen X, Gong T, Liu F, et al. 2015. A new analogue of echinomycin and a new cyclic dipeptide from a marine-derived Streptomyces sp. LS298. Mar Drugs, 13 (11) : 6947–6961 |
| Zhou X, Huang H B, Li J, et al. 2014. New anti-?infective cycloheptadepsipeptide congeners and absolute stereochemistry from the deep sea-?derived Streptomyces drozdowiczii SCSIO 10141. Tetrahedron, 70 (42) : 7795–7801 DOI:10.1016/j.tet.2014.02.007 |
 2016, Vol. 51
2016, Vol. 51